Abstract
A ternary system involving copper-substituted ceria (Cu:CeO2)0.95−y(GO)y(SrTiO3)0.05 nanocomposite where the GO concentration (y) is varied between 0.15 and 0.30. The innovation of this work lies in the purposeful assembly of the nanocomposite using a grinding method, which was optimized through the individual fabrication of Cu-doped CeO2 with different CuO concentrations. In the first, Cu-doped CeO2 with various CuO concentrations (x = 0.0, 0.05, 0.1, 0.2, and 0.3) was first individually fabricated based on a sol–gel auto-combustion strategy to optimize the best ratio. The XRD profiles confirm the successful fabrication of cubic cerium (IV) oxide CeO2 using glycine as a fuel at an annealing temperature of 400 °C for 2 h. AC conductivity was investigated for both Cu-doped CeO2 and the ternary nanocomposite over a wide frequency range (10–1 to 107 Hz) at room temperature. The conductivity showed lower values for CeO2 doped with 0.05, 0.2, and 0.3 wt% CuO, whereas it showed its maximum values upon doping with 0.1 wt% CuO. Interestingly, the conductivity of graphene oxide–doped CeO2 was 103 times higher than that of pure CeO2, i.e., it increased from 1.7 × 10–6 to 2 × 10–3 S/cm. The magnetic-field dependence of MS of the ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites showed a systematic increase in MS as the Cu2+ ion content increased. This strengthens the ferromagnetism in our samples as Cu2+ ion content increases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solid oxide fuel cells (SOFC) are promising power generators that directly convert chemical energy to electricity with low emission and high efficiency, making them a major clean energy conversion technology in the future [1,2,3]. Typically, the cell consists from three main parts: anode, cathode, and electrolyte, but the anode has the most vital role in controlling the overall performance [4, 5]. The anode enriches the oxidation process of the fuel, electrical transport of electrons, and transport of by-products of the electrochemical reaction. Therefore it should have great catalytic activity, moderate electrical conductivity, chemical compatibility, and matching with other SOFC components [6,7,8]. However, there are limitations to be overcome in the commercialization of SOFC, primarily due to the lack of optimal anode materials for high temperature operation (600–900 °C), the region where efficiency is maximized [2, 9]. For instance, the Ni-based cermet anode has high catalytic activity and electrical conductivity at high temperatures. However, the Ni-based electronic conducting network in these anodes can be disrupted by repeated cycling of redox reactions and easy oxidation associated with the coarsening and aggregation of Ni particles. Since that, searching for new functional anode material has been indispensable [10].
To minimize the oxidation and overcome the stability limitations, Ni alloyed with transition metal elements as Co rather than using pure Ni has been introduced. Although alloying has improved durability, cobalt is classified as a critical raw material, i.e., an element of high economic importance but limited supply [11]. Also, oxide anode has drawn much attention for years. Perovskite materials such as doped LaCrO3, doped SrTiO3 [12,13,14], and the double perovskite materials Sr2Mg1−xMoxO6−δ [15] have been developed as SOFC anodes. However, the electronic conductivity of these doped oxides is only on the orders of 1 S/cm under SOFC anode conditions, and the double perovskites are reported as unstable in reducing atmosphere above 900 °C [16, 17]. However, due to the dual magnetic and electrical natures of some of these compounds as SrTiO3, the alignments of magnetic dipole moments helps to increase effective ionic conductivities and diffusivity.
Recently, Cu-doped CeO2 has been reported as a stable ceria-based anode material with higher catalytic activity for carbon gasification and electrical conductivity than pure CeO2. However, this latter needs to be at least one order of magnitude larger for SOFC applications [18]. Improved performances have been obtained by developing composite electrodes of transition metal oxides and graphene. Graphene has almost no bandgap and exhibits remarkable electrical and optical characteristics such as high carrier mobility (2 \(\times\) 105 cm2/V s), high conductivity, unique structure, high mechanical strength, thermochemical stability, and high transparency (∼ 97.7%) [16, 17, 19, 20].
The key idea of the present study is to develop a new functional anode based on the combination of materials with optimized properties to maximize electrical conductivity and enhance durability as well. For that, we have developed, for the first time, a series of (Cu:CeO2)0.95−y(GO)y(SrTiO3)0.05 nanocomposite where (y = 0.15, 0.25 and 0.30) by sol–gel combustion and ball-milling method. To enhance ionic polarizability, SrTiO3 was selected in this study due to its high redox stability and improved performance for fuel oxidation. At the same time, graphene is chemically compatible with SrTiO3-based electrodes and exhibits high ionic conductivity. Moreover, the perovskite is A-site-deficient and alkaline-earth-free to prevent possible superficial phase segregation. The nanocomposite electrodes were studied by various structural, microstructural, magnetic, and electrical techniques to evaluate their potential use as fuel electrodes in solid oxide cells.
2 Materials and methods
2.1 Materials and reagents
Cerous nitrate hexahydrate extra pure AR, 99.9% with the chemical formula [Ce(NO3)3·6H2O] purchased from Loba Chemie PVT. LTD was used to synthesize CeO2 by sol–gel auto-combustion approach. Cupric nitrate trihydrate of 95% purity, with chemical formula Cu (NO3)2·3H2O bought from Loba Chemie PVT. LTD was employed as a Cu2+ source for Cu-doped CeO2 materials. Graphite powder of particle size 0.105 mm, purity of 90%, bought from Nice Chemical (P) LTD, was utilized to fabricate graphene using a modified Hummer’s method. Strontium nitrate, anhydrous, 99.97% with chemical formula Sr(NO3)2 provided from Alfa Aesar and titanium dioxide TiO2 provided by Sisco Research Laboratories were appointed from the production of strontium titanate SrTiO3 nanopowder using sol–gel auto-combustion pathway. Glycine NH2CH2COOH was used in the auto-combustion using sol–gel as a complexing agent/fuel for synthesizing SrTiO3, CeO2, and Cu-doped CeO2 materials.
2.2 Synthesis of pure and Cu-doped CeO2 nanopowder
Using the sol–gel auto combustion technique, CeO2 nanopowder has been customized by mixing a specific ratio of cerous nitrate [Ce(NO3)3·6H2O] with a stoichiometric ratio of glycine in deionized water, 10% hydrogen peroxide as an oxidizing agent was inserted into the solution. After that, the solution was gently thermally treated on a hot plate magnetic stirrer until dry to form a viscous precursor of strontium carboxylic acid complex, which was dried in an oven at 100 °C overnight. Further, the formed powder was ignited in a muffle furnace at 400 °C for 2 h. Otherwise, Cu-doped CeO2 nanopowder was processed with a similar pathway with different Cu contents from 0.05 to 0.30, as recorded in Table 1.
2.3 Preparation of SrTiO3 nanopowder
Anhydrous strontium nitrate was treated with titanium oxide powders in an aqueous solution. Glycine was incorporated into the solution. After that, hydrogen peroxide (10%) was added to the solution to enhance the reactivity of the starting materials. The produced suspension was heated on the hot plate magnetic stirrer until dry. Afterward, the formed powders were dried entirely at 100 °C overnight in an oven. Finally, the formed precursor sample was thermally annealed at 1000 °C for 2 h at the temperature control of 10 °C/min in a static air atmosphere muffle furnace.
2.4 Preparation of Cu:CeO2/GO/SrTiO3 ternary composite
To produce ball-milled ternary nanocomposite, different materials with various ratios given in Table 2 were placed in 130 cm3 agate jars using four 1.5 cm zirconium spheres in a ball mill weighing 25 g. The ball mill equipment was then run for 2 h at 450 rpm [21]. The resultant ball-milled samples were labeled with Composite 1, 2, and 3.
2.5 Physical characterization
X-ray diffraction profiles differentiated the formed products' phase formation based on a Brucker D8-advance using Cu K radiation (λ = 1.5406) with a typical scan range of 2° from 20° to 80° and a scan rate of 2θ/min. The crystallite size of copper-doped CeO2 nanoparticles was obtained utilizing the Debye–Scherrer formula to determine the X-ray diffraction data [22, 23]:
where D is the crystal size, K = 0.9 is a correction factor used to account for particle morphologies, FWHM (full width corrected at half maximum) is the peak's measurement, the wavelength is the measurement, and Bragg angle is the measurement. Transmission electron microscope (JEOL, JEM-1230) operating at 80 kV. A transmission electron microscope (JEOL, JEM-1230) operating at 80 kV was established to assay the synthesized samples' microstructures. The Fourier transform infrared spectroscope (FTIR) was realized using Japan's Jasco JASCO 3600 spectrophotometer. Raman spectra were collected using a confocal Raman microscopy system (LabRAM HR evolution, Horiba Scientific) under excitation with a 532 nm laser. For AC conductivity (σac) measurements, the disc-shaped sample was placed between the two electrodes of the measuring cell connected to the broadband dielectric spectroscopy (BDS), Novo control, Germany. The σac was carried out over a wide frequency range (10–1 to 107 Hz) at room temperature (RT). The σac was carried out over a wide frequency range (10–1 to 107 Hz) at RT and given as a function of the frequency of the applied electric field (f) and the dielectric loss (ε''):
where εo = 8.85 × 10–12 F/m is the permittivity of the vacuum. The error in ε'' amounts to ± 1%. The reproducibility of the measurement was tested by re-measuring σac and ε'' after experimenting once again. The vibrational sample magnetometer (VSM) model, Lake Shore model, 7400 VSM, US, Lake Shore Co., Ltd. Ltd., USA, was employed to measure the magnetic characteristics of the produced samples in an applied magnetic field 20 KOe at RT.
3 Results and discussion
3.1 Cu-doped CeO2
In this study, the sol–gel technique made numerous CeO2 samples based on Cu doping. To optimize the best ratio, these samples' structural characteristics were described using a different molar ratio of (x = 0.0, 0.05, 0.1, 0.2, and 0.3). Figure 1a manifests the XRD forms of Ce1−xCuxO2 (x = 0, 0.05, 0.1, 0.2, and 0.3) tailored based on an auto-combustion method using sol–gel using glycine as a fuel annealed at 400 °C for 2 h. Plainly, All the composed peaks referred to CeO2 as cubic phase according to (JCPDS no. 81-0792) [24]. The diffraction lines related to the lattice planes (111), (200), (220), and (311) facets at 28.5°, 37.3°, 47.5°, and 56.6° are assigned. However, Cu2+ substitution indicates a noticeable decrease in CeO2 crystallinity [25]. The crystallite size of the synthesized powders was evaluated from the highest using (Debye–Scherrer’s equation), the craziest peak (111) of the cerium oxide phase. It was found to be 13 nm for pure CeO2. However, the crystallite size was decreased by the addition of Cu2+ ions. Meanwhile, the crystallite size was increased by further incrementing the Cu2+ ion content. The results imputed that the ionic radius of the Cu2+ ion (0.73 Å) was lower than the ionic radius of the Ce4+ ion (1.01 Å) [26]. The unit cell volume and the lattice constant of Cu subrogated CeO2 were decreased as a Cu2+ ion with a smaller radius was inserted into CeO2 as the result of a contraction of the lattice happened to cause a decrease in the lattice constant. The change in structural parameters is recorded in Table 3.
The Williamson-Hall equation can estimate the crystallite size as predicted in the previous references [27, 28]. Accordingly, the estimated values of D using the Williamson-Hall equation are presented in Table 3. The D value calculated using the Williamson-Hall method is higher than that obtained using the Scherrer equation, where the Williamson-Hall equation considers all diffraction peaks and assumes the brooding of it. That is related to the crystalline size and the lattice strain, which is not considered in the Scherrer equation. The considerable value of D from Williamson-Hall for the 0.30 ratio of Cu-doped CeO2 may be attributed to the large lattice strain due to adding a high percent of Cu.
The microstructure Cu-doped CeO2 nanoparticles are considered by the TEM technique, as given in Fig. 2. The sample of pure CeO2 demonstrates aggregated, nearly well-dispersed spherical NPs with narrow diameters from 4 to 11 nm. The SEAD rings showed the diffraction patterns related to a polycrystalline characteristic of cubic CeO2 was indexed. With an increment of Cu substitution content to 10%, the particle size with the narrow diameters decreased from 2 to 6 nm with a quasi-spherical-like structure. Further, an increase in Cu content to 30%, more agglomeration of the spherical particles, augmenting the particle size to 3–10 nm. However, SEAD patterns evidence the presence of the secondary monoclinic CuO lattice planes with CeO2 at a high concentration of 0.3 Cu2+ ions.
Figure 3a shows diffuse reflectance (DRS) spectra in the wavelength range of 220–2000 nm for pure and Ce1−xCuxO2 (x = 0, 0.05, 0.1, 0.2, and 0.3) samples. As a direct charge transfer, the CeO2 spectrum exhibits the absorption peak at 340 nm at the transition between the O–2p and Ce–4f bands. The reflectance spectrum was changed as the absorption edge shifted to the higher wave number, red-shift, after adding Cu2+ ion. An interfacial polaron effect may cause the red-shift by electron–phonon interaction [29].
Using DRS data, the optical band gap energy (Eg) was estimated. Equations (3) and (4) can be used to compute the Kubelka–Munk equation [21, 30, 31].
where light reflectance is denoted by R, the absorptions (α), and scattering coefficients (S). Another factor, like constant (A), relies on the possibility of the transition. P is a direct or indirect band gap (2 or 1/2), respectively. The Eg values of Cu-substituted CeO2 nanoparticles have been assessed using the Kubelka–Munk absorption function for direct gap semiconductors [21, 32,33,34], evidenced in Fig. 3b and c. A linear correlation between (αhυ)2 and hυ reveals the direct transition nature of Cu-substituted CeO2. The band gap energy was discovered to diminish from 2.99 eV for pure CeO2 to 1.00 eV for Ce0.9Cu0.1O2 with a 10% Cu ratio substituted CeO2. The results can be discussed based on Cu2+ ions with lower ionic radius replaced e Ce4+ ions with bigger ionic radius and the modulation of the required energy for absorption. Subsequently, the Cu2+ ion with 10% content decreased in the band gap energy due to the red-shift for the synthesized as seen by (i.e., increased wavelength).
The conductivity measured at RT for the Ce1−xCuxO2 (x = 0.0, 0.05, 0.1, 0.2, and 0.3) has been investigated first as a function of frequency and then as a function of the composition at a constant frequency value, i.e., 103 Hz. AC conductivity (σac) is a crucial electrical characteristic that controls how well a material conducts electricity when exposed to an electrical field [35, 36]. Furthermore, an increase in the concentration and mobility of ions leads to a conductivity increase [37]. The AC's frequency dependency conductivity (σac) is most often represented by the Jonscher relation [38].
where A and s are constants, [σ(ω)] is the frequency-dependent conductivity recorded with the AC field, and dc is the DC conductivity (σdc). The lines of the conductivity vs. temperature plot are used to estimate the frequency exponent (0 < s ≤ 1), which uses for determining the conduction mechanism [39] as well as the modification of the glass network structure [40]. Generally, Several low-mobility amorphous and crystalline materials are covered by relation (3) [41]. Figure 4a and Table 4 describe the frequency dependence of σac at RT for Ce1−xCuxO2 (x = 0.0, 0.05, 0.1, 0.2, and 0.3). The pure CeO2 conductivity is 0.05, 0.2, and 0.3 wt% doped CeO2 shows lower values, while its maximum values are noticed for those doped with 0.1 wt% CuO. It can be seen from Fig. 4a that the σac increases with increasing frequency because the excitation of electrons increases with the frequency increase, which is the highest value at 0.1 Cu doping. Table 4 compares the σac and optical band gap (Eg) for Ce1−xCuxO2 (x = 0.0, 0.05, 0.1, 0.2, and 0.3) samples, indicating lower values for CeO2 doped with 0.1 wt% CuO is consistent with the same sample's lower band gap value of 1.00 eV. Figure 4b shows the relationship between σac and Eg based on Ce1−xCuxO2 (x = 0.0, 0.05, 0.1, 0.2, and 0.3). It can be deduced that the sample of 0.1 wt. Cu-doped CeO2 showed the lowest value of Eg and correspondingly the highest value of σac,. Therefore, this composition Ce0.9Cu0.1O2 will be chosen, and then we study the effect of introducing GO and/or SrTiO3 and produce a ternary composite.
3.2 Cu:CeO2/GO/SrTiO3 ternary composite
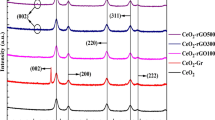
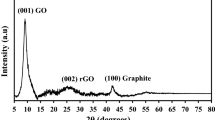
The composite here is fabricated from Ce0.9Cu0.1O2, GO, and SrTiO3. Figure 5a represents the XRD picture of graphene oxide synthesized based on the modified Hammer method [42]. The typical diffraction peaks appeared at 2θ at approximately 12.08° with d-spacing = d = 7.31966 nm, indicating the typical GO peak. Meanwhile, the peak at 2θ of 23.27° with d-spacing 3.81934 nm depicted that the graphite is totally oxidized. Figure 5a indicates the formed crystal structure of strontium titanate SrTiO3 analyzed using XRD patterns synthesized using sol–gel auto-combustion pathway at 1000 \(^\circ{\rm C}\) for 2 h. Obviously, peak intensities associated with cubic SrTiO3 (JCPDS Card No. 35-0734) single-phase material were detected. The characteristic peaks of SrTiO3 evidenced at 2θ angles of 32.4°, 39.9°, 46.4°, 57.7°, and 67.8°, which are related to the crystal planes 110, 111, 200, 211, and 220, respectively, were presented. The size of the generated crystallites SrTiO3 powders was 82.2 nm. Figure 5b evinces the XRD patterns of the ternary composite created by the ball milling route for 2 h. Composite 1 is composed of Cu:CeO2 (0.65):GO (0.30):SrTiO3 (0.05). Composite 2 is composed of Cu:CeO2 (0.70):GO (0.25):SrTiO3 (0.05). While composite 3 is composed of Cu:CeO2 (0.80):GO (0.15):SrTiO3 (0.05). The results demonstrated the peaks corresponding to cerium oxide, graphene oxide, and strontium titanate.
Figure 5c depicts the FT-IR spectrum of Cu-substituted CeO2 nanoparticles from 500 to 4000 cm−1. It is clear that the absorption peaks below 700 cm−1 in Each example demonstrate the Ce–O stretching mode, emphasizing the CeO2 phase being present in all samples [43]. Meanwhile, the peak of absorption occurring at about 1400 cm−1 is related to the atmospheric CO2 non-asymmetric stretching vibration. Meanwhile, the bands centered in all samples at 3000–3500 cm−1 are linked to the O–H vibration of moisture absorption. Meanwhile, the additional 1100 to 1700 cm − 1 peaks are associated with Ce–O stretching [44]. Furthermore, Cu doping observed a slight shift in the absorption bands centers of CeO2. Figure 5c evinces FT-IR spectroscopy of ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites at different stoichiometry ratios. The peak bands at 1000–1300 cm−1 and 1500–1630 cm−1 manifest the entity of C=O and C=C stretching GO vibrational bands, mainly in Composite 1. The prominent peaks correspond to the O–H vibration of water adsorption at 3000 to 3600 cm−1. The band at 1400 cm−1 is assigned to the O–H vibration of graphene oxide. The stretching vibration C–O–C peak occurring at 1210 cm−1 of GO has been indexed [45, 46]. Furthermore, the located peak positions at 2850–2915 cm−1 may be related to CH2 and C– stretching vibration bands related to unburned glycine residue. Meanwhile, the observed central peak is located at 643 cm−1, belonging to the Ti–O stretching vibrations of the SrTiO3 phase in the composite [47]. The distinctive peak at ~ 540 cm−1 is that tall peak of SrTiO3 connected to Sr–Ti–O stretching vibration. Furthermore, the absorption peaks at around 850 cm−1 are due to the existence of Ti–O6 octahedron stretching of SrTiO3 material in the composite. Finally, similar absorption peaks of Cu-substituted CeO2 are also observed [48].
Figure 6a offers the RT Raman spectra of CeO2 and Ce0.9Cu0.1O2 measured within a 0–1000 cm-1 range. A first-order Raman peak (F2g) at 460 cm−1 was observed for each CeO2 and Ce0.9Cu0.1O2, which agrees with the previous reports [49]. It can be noticed that the intensity of F2g peak for Ce0.9Cu0.1O2 is lower than CeO2, indicating the doping by Cu. On the other hand, Fig. 6b displays the Raman spectra of graphene and Composite 2. Apparently, two bands corresponding to the disorder-induced (D-band) and in-phase vibration of graphene (G-bands) were assigned at ~ 1350 and 1585 cm−1 for the GO [50]. At the same time, the main F2g mode band was observed at 455 cm−1, characteristic F2g vibration of CeO2 [51]. Meanwhile, Figure S1 shows the RT Raman spectra of SrTiO3 with weak bands for cubic SrTiO3 at 272, 541, and 794 cm−1 noticed, which the TO3, TO4, and LO4 phonon modes can assign [52]. Raman spectra were used and presented in Figure S2 to identify the electronic interaction. The sharper peak represented the vibration mode of Ce–O at 460 cm−1 (F2g). The band was red-shifted from 460 to 454 cm−1 due to oxygen vacancies, which are linked to constitutional flaws brought on by the absorption of Cu inside the CeO2 lattice, which is present and growing. Plainly, two bands concerning the disorder-induced (D-band) and in-phase vibration of graphene (G-bands) were assigned at ~ 1350 and 1585 cm−1. The stretching vibration of sp2 carbon atoms gives rise to the G band, which correlates with the first-degree scattering of the E2g mode, whereas the stretching vibration of sp3 carbon atoms gives rise to the D band, which results in flaws and disorders. Raman characteristic peaks based on first order, which correspond to the phonon modes of TO3, TO4, and LO4 (271, 543, and 795 cm−1, respectively). At 271 cm−1, the bending vibration of O–Sr–O has been detected. At 543 cm−1, the Ti–O–Ti was noticed for bending vibration mode. At 795 cm−1, the Ti–O stretching type vibration was noticed.
Figure S2 displays TEM micrographs of SrTiO3 and GO samples. It is clear that the microstructure of SrTiO3 nanoparticles exhibited a cubic-like structure, as presented in Figure S2(a). The average grain size was in the range of 7–12 nm. The selected-area electron diffraction (SAED) pattern is described in Figure S2(b). The SAED rings ascribed the cubic SrTiO3 phase was indexed. Figure S2(c) presents the microstructure of GO as a sheet-like structure with a large thickness, a delicate surface, and a rugged edge. SAED pattern in Figure S2(d) displays the diffraction ring related to (002) of the graphitic carbon. Figure 7 represents the morphology Cu-doped CeO2/GO/SrTiO3 nanocomposites at different materials content. The results manifest that the spherical-like nanoparticles of Cu-doped CeO2 and cubic SrTiO3 are well distributed on the graphene nanosheets' surface, as seen in Fig. 7a, d, and g. Figure 7c, f, and i describes the nanoparticle's size and distribution measurements. In this context, the average size was decreased with decreasing the graphene oxide content. The mean grain size was 21.4 nm for composite 1, whereas the primary grain size was 11.38 nm for composite 2, and finally, the grain size of composite 3 was found to be 8.76 nm. SAED patterns showed a clear ring without any distortion in the lattice planes. The SAED rings demonstrate the presence of cubic ceria structure and a more heterogeneous structure in which Cu-doped CeO2 NPs and SrTiO3 were dispersed, evidencing the interaction between ceria and SrTiO3 with GO. Based on the SAED pattern given in Fig. 7b, e, and h, the crystallinity of the composite was changed relating to the insertion of GO material.
Figure S3(e) displays the DRS of ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites in the 200–2000 nm wavelength range. Meanwhile, Cu-doped CeO2 was shifted towards a high wavelength; as a result, the defects in the shape of vacancies in the oxygen were induced by the higher amount of graphene oxide around the Cu-doped CeO2 particles. The absorption peak at 390 nm might be connected to the electrical charge from Ti's 3d orbital to its 2p orbital of O of SrTiO3 in the composite. Owing to Cu-doped CeO2 semiconductor was coupled with narrow bandgap SrTiO3 and graphene oxide semiconductor in composite, which enhanced ability to absorb visible light. The GO exhibits a maximum absorption at 225 nm and a hump at 313 nm, which denote the n-π* transition of the C–O functional groups and the π–π* transition of the C–C bond. The band gap energy Eg value of the composite at different concentrations is demonstrated in Figure S(f). The value is decreased from 2.18 to 2.22, then 2.25 eV relating to the graphene oxide ratio as the result of the Ce–O–C structure formation through the bonding of some unpair p electrons of GO with free electrons of Cu–CeO2 and SrTiO3.
3.3 The frequency dependence
To understand the response of graphene oxide (GO), SrTiO3, and CeO2 compositions, their electrical conductivities profoundly are suggested to be represented at constant frequency value (103 Hz) in one graph as illustrated in Fig. 8a and listed in Table 5. Compared to CeO2 compositions, GO exhibited the highest conductivity value (σac = 10–2 S/cm), whereas SrTiO3 showed the lowest value (σac = 7.5 × 10–9 S/cm). Since adding SrTiO3 to the composite is planned to enhance the chemical stability, we must keep a minimum content of SrTiO3 as low as 0.05 wt% into the composite to eliminate the possible increase in conductivity. Also, GO–doped CeO2 showed much higher conductivity values than those doped with CuO; the duel composite of CeO2 and 0.25 wt% GO showed a much higher conductivity than all Cu-doped CeO2 composite samples.
a AC conductivity at 103 Hz for the graphene oxide (GO), strontium titanate (SrTiO3), CuxCe1−xO2 (x = 0.0, 0.05, 0.1, 0.2, and 0.3), and Ce1−(y+0.5)Gy + Sr0.5 (y = 0.15, 0.25, and 0.3). b The frequency dependence of AC conductivity (σac) for CeO2, SrTiO3, and Graphene oxide (GO) at RT. c The frequency dependence of AC conductivity (σac) for 0.1 wt% CuO-doped Ce1−(y+0.05)Gy + Sr0.05 (y = 0.15, 0.25, and 0.3) at RT
Figure 8b manifests the frequency-dependent conductivity of the pure CeO2 in comparison with that recorded for the graphene oxide (GO) and strontium titanate (SrTiO3). It is evident that the conductivity of both CeO2 and SrTiO3 increased with increasing frequency and also obeys Jonscher Equation. In contrast, the conductivity of GO shows frequency independence over the whole frequency window, leading to DC conductivity only. Further, it shows much higher values as compared to CeO2 and SrTiO3. Figure 8c offers the frequency-dependent conductivity of 0.1 wt% CuO-doped CeO2 compared to those doped with GO (0.15, 0.25, and 0.3 wt%) and SrTiO3 (0.05 wt%). As evident, the conductivity of GO-doped CeO2 ceramics is frequency independent up to 106 Hz, then significantly increases following the universal power law at higher frequencies. It has been found that the GO-doped CeO2 ceramics showed higher conductivity values than Cu-doped CeO2 ceramics. Notably, the highest conductivity (σac = 2 × 10–3) has been found to composite 2 of Cu:CeO2 (0.70):GO (0.25):SrTiO3 (0.05), enabling a novel mechanism for the production of anode materials and a novel fuel cell principle better than reported through literature [53]. The possible underlying mechanism involves the formation of a surface oxygen-deficient layer and core–shell architecture of GO-doped CeO2. Also. for these composites, σac increases with increasing frequency because the excitation of electrons increases with the frequency increase. Further, the observed conductivity decrease occurs at two different rates; it seemed to be nearly perpetual in the low-frequency range, leading to DC conductivity (σdc), while it considerably increased following the universal power law at higher frequencies (ωs) [54]. This might be due to the hopping of free-charge carriers between different localized states.
3.4 Magnetic properties
Undoped CeO2 and Cu-doped CeO2 samples' magnetic-field dependency on magnetization (M–H curve) is depicted in Fig. 9a from vibrating sample magnetometer (VSM) measurements at RT. Remarkably, all samples showed very closed M-H curves with coercive fields less than 5 Oe. Also, the average crystallite size obtained from X-ray diffraction analysis (Table 3) is relatively equivalent to the average particle size obtained from TEM analysis (Fig. 2); indicating the formation of single magnetic-domain structures and the presence of superparamagnetic states in our samples. Notably, the undoped CeO2 nanoparticles exhibited a mixed behavior of weak ferromagnetism at low and diamagnetism at high fields. It also showed a relatively higher Ms of 0.003 emu/g at ≤ 5 kOe than reported in the literature [55, 56]. This is a promising result because the bulk CeO2 is insulating in its origin with Ce4+ in the 4f0 configuration. Magnetic order in insulating materials originates from owing to the superexchange, and antiferromagnetic ordering exists interaction. However, CeO2 nanoparticles may show some ferromagnetism because oxygen vacancies can be easily formed during the annealing and crystallization process. Similar outcomes have also been attained in other works [37]. The group showed weak ferromagnetic behaviors, with MS ≈ 0.0019 emu/g at 5 kOe in CeO2 and some similar oxides, resulting from the superexchange interactions across oxygen vacancies. Regarding Cu-doped CeO2, MS increased as Cu content increased, and the most significant MS was noted for x = 0.3, with a value of approximately 0.018 emu/g at 5 KOe. These results confirmed the enhancement of the superexchange interactions of CeO2 nanoparticles. Based on the electronic configuration of copper 3d104s1, we expect the presence of Cu1+ and Cu2+ ions due to the possible formation of 3d10 and 3d9 configurations, respectively. Since the d-electrons are paired in the 3d10 configuration, the Cu1+ ion does not possess a magnetic moment. However, the presence of an unpaired electron in the outer shell of the Cu2+ ion with a 3d9 configuration contributes to the net magnetization. This strengthens the superparamagnetism in our samples as Cu2+ ion content increases. Figure 9b shows the magnetic-field dependence of MS of the ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites. Results showed a systematic increase in MS as the Cu2+ ion content increased.
3.5 Ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites in fuel cell industry
Platinum-based catalysts: Platinum (Pt) is a widely used catalyst material in fuel cells due to its excellent electrocatalytic activity. Compared to Pt-based catalysts, the ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites offer the advantage of lower cost, as Cu is a more economical alternative to platinum. However, Pt-based catalysts still exhibit superior performance in terms of catalytic activity [57]. Nickel-based catalysts: Nickel (Ni) is another commonly used catalyst material in fuel cells. Compared to nickel-based catalysts, the ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites may provide improved stability and durability, as copper oxide (CuO) has been shown to possess enhanced resistance to corrosion and sintering. This could lead to a longer lifespan and improved fuel cell performance [58]. Conductive Supports: GO is often employed as a conductive support material in fuel cell electrode designs due to its high electrical conductivity [59]. The ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites leverage this advantage by incorporating GO. This composite structure facilitates efficient charge transfer and enhances the overall electrical conductivity of the electrode material. CeO2: The addition of cerium oxide in the nanocomposites provides various benefits. CeO2 possesses high oxygen storage capacity and can facilitate oxygen transport within the fuel cell. It also exhibits excellent redox properties, aiding in the oxidation and reduction reactions during fuel cell operation. Incorporating copper into the CeO2 matrix further enhances its catalytic and electronic properties. Strontium titanate (SrTiO3):SrTiO3 is a perovskite material known for its high dielectric constant and stability. By incorporating SrTiO3 into the nanocomposites, the material gains enhanced structural integrity and improved resistance to mechanical stress during fuel cell operation. The presence of SrTiO3 also contributes to the overall conductivity and stability of the nanocomposites. While the ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites offer advantages such as lower cost, improved stability, and enhanced conductivity compared to certain materials used in fuel cells, they may not match the catalytic activity of platinum-based catalysts. However, the unique combination of copper doping, graphene oxide support, cerium oxide, and strontium titanate in the nanocomposites presents an intriguing avenue for further exploration and optimization in fuel cell applications. It is important to note that these comparisons are general, and further experimental evaluations and performance testing are necessary to provide more precise and quantitative assessments of the ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites compared to other materials in the fuel cell industry.
4 Conclusions
It has been designed to create Ce1−xCuxO2 nanostructures using the sol–gel process. The effect of the Cu ions concentration (0.00 ≤ x ≥ 0.3) on the structure and morphology of the CeO2 nanocrystals was investigated using TEM, XRD, and optical band gap. In the crystal structure of cerium oxide, XRD revealed that Cu ions were utterly soluble. Then, a ternary composite of (Cu:CeO2)0.95−y(GO)y(SrTiO3)0.05 nanocomposite where (y = 0.15, 0.25, and 0.30) has been carefully built using the grinding technique. AC conductivity, magnetic properties, optical properties, morphological structures, and XRD for graphene oxide, SrTiO3, Cu, and/and GO-doped CeO2 ceramics have indeed been examined concerning frequency and composition. Experimental results showed that the conductivity increases with increasing frequency and obeys the universal power law, i.e., Jonscher Equation. Interestingly, the conductivity of the graphene oxide doped CeO2 has been dramatically enhanced, which can be helpful in the fuel cell industry. Regarding Cu-doped CeO2, MS increased as Cu content increased, and the most significant Ms was noted for x = 0.3, with a value of approximately 0.018 emu/g at 5 KOe. Finally, results showed that the magnetic-field dependence of MS of the ternary Cu-doped CeO2/GO/SrTiO3 nanocomposites increased in MS as the Cu2+ ion content increased.
Data availability
Some or all data of this study are available from the corresponding author upon reasonable request.
References
J. Zhang, S. Ricote, P.V. Hendriksen, Y. Chen, Advanced materials for thin-film solid oxide fuel cells: recent progress and challenges in boosting the device performance at low temperatures. Adv. Funct. Mater. 32, 2111205 (2022)
G. Chasta, M.S. Dhaka, A review on materials, advantages, and challenges in thin film based solid oxide fuel cells. Int. J. Energy Res. 46, 14627–14658 (2022)
B. Ramasubramanian, R.P. Rao, V. Chellappan, S. Ramakrishna, Towards sustainable fuel cells and batteries with an AI perspective. Sustainability 14, 16001 (2022)
T.M. Gür, Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ. Sci. 11, 2696–2767 (2018)
Z. Jiang, A.L. Snowdon, A. Siddiq, A. El-Kharouf, R. Steinberger-Wilckens, Optimization of a ScCeSZ/GDC bi-layer electrolyte fabrication process for intermediate temperature solid oxide fuel cells. Ceram. Int. 48, 32844–32852 (2022)
V. Sarıboğa, M.F. Öksüzömer, Cu-CeO2 anodes for solid oxide fuel cells: determination of infiltration characteristics. J. Alloys Compd. 688, 323–331 (2016)
J. Zamudio-García, J.M. Porras-Vázquez, E.R. Losilla, D. Marrero-López, LaCrO3–CeO2-based nanocomposite electrodes for efficient symmetrical solid oxide fuel cells. ACS Appl. Energy Mater. 5, 4536–4546 (2022)
N. Momin, J. Manjanna, S. Kobayashi, S.T. Aruna, S.S. Kumar, G.P. Nayaka, Synthesis and ionic conductivity of calcium-doped ceria relevant to solid oxide fuel cell applications. Mater. Adv. 3(23), 8780–8791 (2022)
S. Jo, H.G. Jeong, Y.H. Kim, D. Neagu, J.-H. Myung, Stability and activity controls of Cu nanoparticles for high-performance solid oxide fuel cells. Appl. Catal. B 285, 119828 (2021)
Y. Liu, Z. Shao, T. Mori, S.P. Jiang, Development of nickel based cermet anode materials in solid oxide fuel cells—now and future. Mater. Rep.: Energy 1, 100003 (2021)
K. Matsumoto, Y. Tachikawa, S.M. Lyth, J. Matsuda, K. Sasaki, Performance and durability of Ni–Co alloy cermet anodes for solid oxide fuel cells. Int. J. Hydrog. Energy 47, 29441–29455 (2022)
M. Ke, W. Wang, X. Yang, B. Li, H. Li, Doped strontium titanate anode for solid oxide fuel cells: electrical and sintering behavior. Ceram. Int. 48, 8709–8714 (2022)
M.Y. Shah, Y. Lu, N. Mushtaq, S. Rauf, M. Yousaf, M.I. Asghar, P.D. Lund, B. Zhu, Demonstrating the potential of iron-doped strontium titanate electrolyte with high-performance for low temperature ceramic fuel cells. Renew. Energy 196, 901–911 (2022)
J. Cavazzani, E. Squizzato, E. Brusamarello, A. Glisenti, Exsolution in Ni-doped lanthanum strontium titanate: a perovskite-based material for anode application in ammonia-fed solid oxide fuel cell. Int. J. Hydrog. Energy 47, 13921–13932 (2022)
Y.-H. Huang, G. Liang, M. Croft, M. Lehtimaki, M. Karppinen, J.B. Goodenough, Double-perovskite anode materials Sr2MMoO6 (M = Co, Ni) for solid oxide fuel cells. Chem. Mater. 21, 2319–2326 (2009)
A. Tabish, H. Patel, A. Mani, J. Schoonman, P. Aravind, Effect of H2S and HCl contaminants on nickel and ceria pattern anode solid oxide fuel cells. Electrochim. Acta 423, 140592 (2022)
K. Sood, P. Tiwari, S. Dhall, S. Basu, Understanding electrocatalytic activity of titanium and samarium doped ceria as anode material for solid oxide fuel cells. J. Electroanal. Chem. 925, 116902 (2022)
I. Díaz Aburto, J. Hidalgo, E. Fuentes Mendoza, S. González Poggini, H. Estay, M. Colet Lagrille, Mo, Cu-doped CeO2 as anode material of solid oxide fuel cells (SOFCs) using syngas as fuel. J. Electrochem. Sci. Technol. 12, 246–256 (2021)
R.M.N. Javed, A. Al-Othman, M. Tawalbeh, A.G. Olabi, Recent developments in graphene and graphene oxide materials for polymer electrolyte membrane fuel cells applications. Renew. Sustain. Energy Rev. 168, 112836 (2022)
P. Tang, Z. Deng, Y. Zhang, L.X. Liu, Z. Wang, Z.Z. Yu, H.B. Zhang, Tough, strong, and conductive graphene fibers by optimizing surface chemistry of graphene oxide precursor. Adv. Funct. Mater. 32, 2112156 (2022)
A.M. Elseman, M.M. Rashad, A.M. Hassan, Easily attainable, efficient solar cell with mass yield of nanorod single-crystalline organo-metal halide perovskite based on a ball milling technique. ACS Sustain. Chem. Eng. 4, 4875–4886 (2016)
U. Holzwarth, N. Gibson, The Scherrer equation versus the’Debye-Scherrer equation’. Nat. Nanotechnol. 6, 534–534 (2011)
M.M. Rashad, A.M. Elseman, A.M. Hassan, Facile synthesis, characterization and structural evolution of nanorods single-crystalline (C4H9NH3)2PbI2X2 mixed halide organometal perovskite for solar cell application. Optik 127, 9775–9787 (2016)
X.-J. Yang, W. Shu, H.-M. Sun, X.-B. Wang, J.-S. Lian, Preparation and photocatalytic performance of Cu-doped TiO2 nanoparticles. Trans. Nonferrous Metals Soc. China 25, 504–509 (2015)
L. Li, Y. Zhan, Q. Zheng, Y. Zheng, X. Lin, D. Li, J. Zhu, Water–gas shift reaction over aluminum promoted Cu/CeO2 nanocatalysts characterized by XRD, BET, TPR and cyclic voltammetry (CV). Catal. Lett. 118, 91–97 (2007)
Z.-Y. Pu, X.S. Liu, A.P. Jia, Y.L. Xie, J.Q. Lu, M.F. Luo, Enhanced activity for CO oxidation over Pr-and Cu-doped CeO2 catalysts: effect of oxygen vacancies. J. Phys. Chem. C 112, 15045–15051 (2008)
S.K. Abdel-Aal, A.S. Abdel-Rahman, Graphene influence on the structure, magnetic, and optical properties of rare-earth perovskite. J. Nanopart. Res. 22, 267 (2020)
S.K. Abdel-Aal, M.F. Kandeel, A.F. El-Sherif, A.S. Abdel-Rahman, Synthesis, characterization, and optical properties of new organic–inorganic hybrid perovskites [(NH3)2(CH2)3]CuCl4 and [(NH3)2(CH2)4]CuCl2Br2. Phys. Status Solidi (a) 218, 2100036 (2021)
K. Kumari et al., Engineering the optical properties of Cu doped CeO2 NCs for application in white LED. Ceram. Int. 46, 7482–7488 (2020)
Y. Elbashar et al., Optical spectroscopic analysis of bandpass absorption glass filter based on vanadium copper oxide for laser safety applications. Nonlinear Opt. Quantum Opt.: Concepts Mod. Opt. 51, 195–212 (2019)
A.M. Elseman, L. Luo, Q.L. Song, Self-doping synthesis of trivalent Ni2O3 as a hole transport layer for high fill factor and efficient inverted perovskite solar cells. Dalton Trans. 49, 14243–14250 (2020)
M.L. Myrick et al., The Kubelka-Munk diffuse reflectance formula revisited. Appl. Spectrosc. Rev. 46, 140–165 (2011)
M. Abou-Mesalam, M. Abass, A. Ibrahim, A. Elseman, A. Hassan, Tunable optical and dielectric properties of polymeric composite materials based on magneso-silicate. Bull. Mater. Sci. 42, 1–11 (2019)
A.E. Abd El-Samad, N. Gad, M. El-Aasser, M.M. Rashad, A.M. Elseman, Optoelectronic investigation and simulation study of zinc and cobalt doped lead halide perovskite nanocrystals. Sol. Energy 247, 553–563 (2022)
J.K. Nelson, J.C. Fothergill, Internal charge behaviour of nanocomposites. Nanotechnology 15, 586 (2004)
A.M. Nassar, E.F. Abo Zeid, A.M. Elseman, N.F. Alotaibi, A novel heterometallic compound for design and study of electrical properties of silver nanoparticles-decorated lead compounds. New J. Chem. 42, 1387–1395 (2018)
M.T. Ramesan, Enhanced dielectric properties, thermal stability and ammonia sensing performance of poly (diphenylamine)/zinc oxide nanocomposites via one step polymerization. J. Appl. Polym. Sci. 139, e52913 (2022)
H. El-Mallah, AC electrical conductivity and dielectric properties of perovskite (Pb, Ca) TiO3 ceramic. Acta Phys. Pol. A 122, 174 (2012)
S. Ojha, M.S. Ali, M. Roy, S. Bhattacharya, Hopping frequency and conductivity relaxation of promising chalcogenides: AC conductivity and dielectric relaxation approaches. Mater. Res. Express 8, 085203 (2021)
Y.-H. Rim, C.-G. Baek, Y.-S. Yang, Characterization of Ionic Transport in Li2O-(Mn: Fe)2O3-P2O5 Glasses for Li batteries. Materials 15, 8176 (2022)
N. Mott, E. Davis, Electronic process in non-crystalline materials (Oxford University Press, Oxford, 1971)
L. Shahriary, A.A. Athawale, Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2, 58–63 (2014)
P.T. Shibeshi, D. Parajuli, N. Murali, Study of Fe-doped and glucose-capped CeO2 nanoparticles synthesized by co-precipitation method. Chem. Phys. 561, 111617 (2022)
M. Keerthana, T.P. Malini, R. Sangavi, Efficiency of cerium oxide (CeO2) nano-catalyst in degrading the toxic and persistent 4-nitrophenol in aqueous solution. Mater. Today: Proc. 50, 375–379 (2022)
T.A. Saleh, K. Haruna, A.-R.I. Mohammed, Octanoate grafted graphene oxide as an effective inhibitor against oil well acidizing corrosion. J. Mol. Liq. 325, 115060 (2021)
T. Heidarzadeh, N. Nami, D. Zareyee, Application of (MWCNTs)-COOH/CeO2 hybrid as an efficient catalyst for the synthesis of some nitrogen-containing organic compounds. Inorg. Nano-Metal Chem. 52, 1173–1182 (2022)
Y. Liu, M. Yao, X. Yao, Enhancement of energy storage density achieved in Bi-modified SrTiO3 thin films by introducing a TiO2 layer. J. Alloys Compd. 882, 160668 (2021)
M.F. Kandeel, S.K. Abdel-Aal, A.F. El-Sherif, H.S. Ayoub, A.S. Abdel-Rahman, in IOP conference series: materials science and engineering, vol. 610, (IOP Publishing, Bristol, 2019), p.012063
W. Weber, K. Hass, J. McBride, Raman study of CeO2: second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 48, 178 (1993)
M. Kasztelan et al., Ammonia modified graphene oxide–gold nanoparticles composite as a substrate for surface enhanced Raman spectroscopy. Appl. Surf. Sci. 554, 149060 (2021)
J. Zimou et al., Structural, morphological, optical, and electrochemical properties of Co-doped CeO2 thin films. Mater. Sci. Semicond. Process. 135, 106049 (2021)
M. Ganapathy, Y. Hsu, J. Thomas, C.T. Chang, V. Alagan, Co-catalyst free SrTiO3 nano-cube for efficient photocatalytic hydrogen production. J. Mater. Sci. 56, 18976–18988 (2021)
S. Sumi, P.P. Rao, M. Deepa, P. Koshy, Electrical conductivity and impedance spectroscopy studies of cerium based aeschynite type semiconducting oxides: CeTiMO6 (M=Nb or Ta). J. Appl. Phys. (2010). https://doi.org/10.1063/1.3481389
M. Lira-Cantu, K. Norrman, J.W. Andreasen, F.C. Krebs, Oxygen release and exchange in niobium oxide MEHPPV hybrid solar cells. Chem. Mater. 18, 5684–5690 (2006)
A. Sundaresan, R. Bhargavi, N. Rangarajan, U. Siddesh, C. Rao, Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys. Rev. B 74, 161306 (2006)
S.-Y. Chen, Y.-H. Lu, T.-W. Huang, D.-C. Yan, C.-L. Dong, Oxygen vacancy dependent magnetism of CeO2 nanoparticles prepared by thermal decomposition method. J. Phys. Chem. C 114, 19576–19581 (2010)
S. Samad et al., Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int. J. Hydrog. Energy 43, 7823–7854 (2018)
D. Khalafallah, M. Zhi, Z. Hong, Development trends on nickel-based electrocatalysts for direct hydrazine fuel cells. ChemCatChem 13, 81–110 (2021)
H. Huang, X. Wang, Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2, 6266–6291 (2014)
Acknowledgements
This work was financially supported by the Academy of Scientific Research and Technology (ASRT). The Science, Technology & Innovation Funding Authority (STDF), Egypt (Grant No. 25250), supported this work. Thanks to the Central Metallurgical Research & Development Institute for supporting the synthesis and measuring instruments.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
FH: conceptualization, methodology, AME: data curation, investigation, formal analysis, writing—original draft preparation, MR: resources, writing—original draft preparation, supervision, RM: project administration, SAS: supervision, MMR: writing—original draft preparation, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hossam, F., Elseman, A.M., Rasly, M. et al. Observation of structural, optical, electrical and magnetic properties of ternary copper-doped CeO2/GO/SrTiO3 nanocomposites. J Mater Sci: Mater Electron 34, 1513 (2023). https://doi.org/10.1007/s10854-023-10901-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10901-0