Abstract
Nanostructured 0.52 Bi2O3–0.18 Fe2O3–0.30 PbTiO3 (BFPT) mol% sample was prepared via a high-energy planetary ball mill. To obtain nanostructured materials, the mixture was mechanically milled for 1, 2, 5, and 25 h. The heat treating process was applied to the milled BFPT sample at 673, 873, and 1073 K for 5 h. The amorphous natures of the as-milled and crystallized formed phases of the heat treated samples are examined using the X-ray diffraction (XRD) technique. HRTEM and XRD were used to confirm the amorphous nature and nanocrystallization of the milled and the heat treated BFPT samples, respectively. The effect of the heat-treatment temperature of BFPT samples on their dc electrical conductivity (σ), density (ρ), and oxygen molar volume (Vm) was studied. The results of the thermoelectric power indicate a p-type semiconducting behavior of the BFPT samples. The high temperature (above θD/2) dependent on conductivity was explicated by the small polaron hopping (SPH) model. While Greaves’ variable range hopping (VRH) model was studied at intermediate temperatures. The physical parameters determined from the best fits of these models were thought to be reasonable and consistent with the BFPT samples. The hopping carrier mobility was found to be the dominant factor for determining the conductivity in the BFPT samples. The electronic transport between Fe ions was primarily responsible for the conduction, which was shown to obey the adiabatic SPH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For many years, bismuth ferrite (BF) is well-known that exhibits two main phases: perovskite-type BiFeO3 and sillenite-type Bi25FeO40 [1]. As reported in the literature [2], the phase stabilization temperature range of pure BiFeO3 is narrow, so it is difficult to prepare it. Therefore, the apparition of Bi25FeO40 and Bi2Fe4O9 phases is most common when high temperatures are applied to xBF-(1-x)PT systems(x close to 1 mol% and PT refers to PbTiO3) [3]. On the other hand, BiFeO3 may decompose to a bismuth-rich phase of composition Bi25FeO40 and Bi2Fe4O9 phases under certain conditions [4]. The crystallization of Bi25FeO40 occurs in the cubic structure with cell parameter ~ 1.018 nm and space group I23 [5]. However, the presence of Bi25FeO40 and Bi2Fe4O9 phases causes high leakage current in the sample, resulting degradation in the ferroelectric behavior. Although, it is found that iron-sillenite Bi25FeO40 is of great interest owing to its piezoelectric and electro-optic properties [5, 6]. In order to improve the electrical properties, ABO3 (i.e., A and B are cations, and O is an anion) perovskite compounds can be introduced. PbTiO3 (PT) is a tetragonal distorted perovskite structure with a space group of P4mm and a Curie temperature of ∼ 763 K [7]. Because materials that depend on BF are focused on the role of strong electron–phonon coupling in the generation of polarons, it seems to be fascinating to explore the details of electrical conductivity and the small polaron hopping (SPH) mechanism [8]. The BFPT solid solution system is characterized by a high Curie temperature (Tc∼ 905 K) and weak-ferromagnetic at high temperatures (Néel temperature “TN” ∼ 650 K) [7, 9]. Therefore, it is a promising candidate to be applied in high temperature devices, with potential applicability in piezoelectric sensors, electromechanical transducers, and actuators [10]. Since, these devices can be operated at temperatures above 473 K [9].
Mechanical milling is a solid-state reaction technique that includes cold welding and fracturing of powder particles [11]. The powder particles are continuously impacted by the balls in the vial, so fractured particles and cold welding between the particles are repeated during the ball-milling process [11, 12]. Therefore, it is used for preparing nanostructured materials [12]. At higher milling intensities, it is thought that the collision energy between the powdered sample, milling vial, and balls will be sufficiently high for the formation of higher defect concentrations in the powder and hence helping in amorphization [13]. It is well-known that the mechanical milling method assists in the formation of perovskite-type oxides through both mechanical activation of the reactants and further annealing at moderate temperatures, or through room temperature mechanical milling [3].
The transport mechanism in transition metal oxide (TMO) systems has been explicated using the polaron hopping [14]. In the BFPT system, the hopping of electrons from low to higher valence states between Fe2+ and Fe3+ ions is responsible for the conduction process. Strong electron–phonon interaction is thought to be responsible for the formation of a quasiparticle called a polaron [15, 16]. The electrical conduction mechanism that happens in the BFPT system is termed SPH model. The electrical conductivity of these nanomaterials is highly dependent on the distance between the Fe ions and the local interaction of an electron with its surroundings [16].
The main purpose of this work is, first, to prepare nanostructured 0.52 Bi2O3–0.18 Fe2O3–0.30 PbTiO3 (BFPT) mol% samples via high energy mechanical milling technique. Since, to the best of our knowledge, no detailed studies have been performed on the structure and physical properties of the BFPT system using high-energy planetary ball mill method. Second, study the effect of heat treating temperature on the nanostructure and electrical properties of the milled and heat treated BFPT samples. Third, to investigate the composition-dependent structural and transport properties of BFPT samples from the point of view of different models.
2 Experimental
Nanostructured 0.52 Bi2O3–0.18 Fe2O3–0.30 PbTiO3 (BFPT) mol% sample was prepared via mechanical milling. Reagent-grade Bi2O3 (99%), Fe2O3 (99%) (Loba Chemie), and PbTiO3 (99%) (Aldrich Chemical) powders were used as raw materials. The mixture was mechanically milled at different milling times (1, 2, 5, and 25 h) in a high energy planetary ball mill by using hard stainless steel balls with a balls-to-powder weight ratio 20:1 and rotation speed of 350 rpm. After every 30 min, we stopped the milling for 10 min to cool down the system. After milling, the sample was pressed into 4 mm-diameter pellets under 900 MPa using a uniaxial press. Then, the pellet samples were heat-treated at different temperatures 673, 873, and 1073 K for 5 h in an automatic temperature-controlled furnace. The measurement of heating time begins when the furnace reaches the heat-treatment temperature. All of the heat-treated samples were cooled in the furnace.
X-ray diffraction patterns (XRD) of the as-milled and heat treated powders were obtained using the Siemens D5000 X-ray diffractometer with Cu Kα radiation (λ = 0.154 nm) under an accelerating voltage of 40 kV and a current of 30 mA. The diffractometer was operated within the range of 10°< 2θ < 70° sing a step size of 0.05 deg/sec. The structure and particle size of the BFPT samples were examined by high-resolution transmission electron microscopy (HRTEM) using “JEOL JEM-2100”.
The density (ρ) of the prepared samples was measured using toluene as the immersion liquid at room temperature. Density is commonly measured using the liquid displacement method, which is based on Archimedes’ principle. Each sample was measured three times to calculate the average density using the following formula:
where Wa, Wl, and ρl represent the weight in the air, the weight in toluene, and the density of toluene, respectively. All the prepared samples were measured utilizing a digital balance. By using the molecular weight of the measured samples (Mwt), the molar volume (Vm) could be determined from the relation:
Samples were polished to get parallel surfaces with thickness ranging from 1.3 to 1.6 mm. For electrical measurements, silver paste is deposited as two-point probes on both surfaces of the polished samples. The thermoelectric power measurements (TEP) of the prepared samples were performed by using two copper electrodes and two K-type digital thermocouples connected to the samples. TEP measurements were carried out in the temperature range of 310–470 K. The dc conductivity (σ) of the prepared samples was measured using a KEITHLEY 485 picoammeter, in the range of 310–425 K. The I-V characteristic electrode was verified.
3 Results and discussion
3.1 X-ray diffraction analysis (XRD)
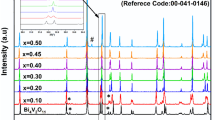
Figure 1a displays the XRD patterns of Bi2O3, Fe2O3, PbTiO3, BFPT as-mix, and the BFPT powder after high-energy milling at various milling times of 1, 2, 5, and 25 h. The XRD patterns of the as-received pure Bi2O3, Fe2O3, PbTiO3 and the as-mix BFPT powder refer to the purity of the raw materials. As illustrated, after 1 h of high-energy ball milling, the characteristic oxide peaks have shown a significant reduction in intensity due to severe plastic deformation. The amorphization process begins once the powders have been subjected to a definite number of critical loading events that depend on the impact energy [11, 17]. The unusual reactivity recognized was attributed to working conditions away from equilibrium, leading to severe plastic deformations and local excited states [11, 17]. After 2 h of milling, only a very broad peak (centered at 2θ =29.6°) appeared because of the complete amorphization of the BFPT sample. Since the broadening peak confirmed that the particles' size was reduced to nano-scale and the lattice strain was improved. We have duplicated the milling time five times (from 5 to 25 h) to observe amorphization changes or recrystallization processes, which are both possible in some systems. However, as shown in Fig. 1a, there is no change. Therefore, the BFPT sample milled for 5 h has been taken to be heat treated at 673, 873, and 1073 K for 5 h. Following heat treating, the BFPT samples were examined by using XRD, as displayed in Fig. 1b. The XRD peaks observed indicate that the diffraction patterns of the as-heat treated BFPT at 673 K can be attributed to cubic Bi25FeO40, cubic Bi12TiO20, and tetragonal Pb2Bi4Ti5O18 phases. At 873 K, new diffraction peaks observed at 2θ = 22.97°, 31.41°, 39.22° and 57.48 °with the (100), (101), (111), and (211) planes are ascribed to the tetragonal PbTiO3 phase, respectively. As observed, there is a small shift in the positions of the peaks of the XRD patterns owing to accumulated strain during the mechanical milling. The JCPDS cards were used to verify these four phases, as shown in Fig. 1b. On further increasing the heat treating temperature, the intensities of the peaks corresponding to the four mentioned phases decreased for the powder heated at 1073 K. This crystallinity reduction is due to the efThe measured d-spacing valuefect of the low melting point of the Bi-rich phase. According to recent papers [2, 4], heat treating temperatures higher than 1048 K, may lead to the formation of a Bi-rich liquid phase.
The average lattice strain (ε) and crystallite sizes (D) of the nanostructured BFPT samples can be calculated using the Williamson–Hall relation [18]:
where \(\beta \) is the full width half maximum (FWHM), \(\uptheta \) is the diffraction angle, k = 0.89 is the Scherrer constant, and λ is the Cu target wavelength. The variation of lattice strain (ε) and crystallite sizes (D) of the nanostructured BFPT samples is presented in Table 1. Therefore, the variation of the crystallite size may be ascribed to the change in phase with milling time over the agglomeration and diffusion processes, as reported previously in [19, 20].
3.2 High-resolution transmission electron microscope (HRTEM)
We have used high-resolution transmission electron microscopy (HTEM) to examine the particle size and interplanar distance (d-spacing) of the BFPT powders milled for 5 h and heat treated at 873 K, as represented in Figs. 2, 3, respectively. Figures 2a, b) show the BFPT powder milled for 5 h at 50 nm and 10 nm scales, respectively. As illustrated, there is a particle agglomeration of the ball-milled powder leading to form the large particles, hence, it is difficult to observe. The selective area electron diffraction (SAED) pattern that is represented as an inset in Fig. 2 shows only a halo without spots. This also confirms the amorphous nature of the 5 h milled nanostructured BFPT. The electron micrograph at 100 nm scale, recorded for the BFPT powder heat treated at 873 K for 5 h (Fig. 3a), shows the particle crystallization. The average particle size is ~ 46.3 nm. That is nearly equal to that measured from XRD (44.43 nm). Figure 3b represents the interplanar distance (d-spacing) of these particles. The measured d-spacing value is 0.321 nm, which is very similar to the d-spacing value of the Bi25FeO40 and/or Bi12TiO20 plane (310). The nanocrystalline nature of the BFPT powder heat treated at 873 K is confirmed by the SAED pattern represented in Fig. 3 (inset). However, spots in this pattern suggest the presence of nanocrystallization. Therefore, we deduced that the results achieved by HRTEM and XRD are well agreed upon.
3.3 Density and oxygen molar volume
The measurement of bulk density is mostly the simplest physical property [21]. Although it would be a very informative property if the material’s structure could be properly defined [21]. The bulk density of the prepared pellets is impacted by the structure formed, compactness, geometric configuration changes, and pellet dimension [22]. Figure 4 investigates the effect of the heat treatment (HT) temperature on the density (ρ) and oxygen molar volume (Vm) for the nanostructured BFPT samples. As displayed in the figure, the density increases with the increasing HT temperature to 873 K; after that, it decreases when the BFPT sample is heat-treated at 1073 K. While the oxygen molar volume exhibits the opposite behavior. As shown in Table 2, as the temperature of the HT increases, the values of the density follow the same behavior as the crystalline size. This is due to the fact that the degree of crystallinity changes with the HT temperatures, which results in a change in the mass per unit volume of these samples.
From the density measurements, we can calculate the concentration of iron ions (N) by using formula [23]:
where p denotes to the weight percentage of atoms and NA denotes to the Avogadro’s number.
Accordingly, the mean distance between iron ions (R) is given by [16]:
The evaluated values of ρ, Vm, R and N are obtained in Table 2.
3.4 Thermoelectric power properties (TEP)
In Fig. 5, the Seebeck coefficient values (S) are plotted as a function of temperature for BFPT samples. The Seebeck coefficient is given by a measurement of the ratio of the sample voltage (∆V) to the temperature differences along the sample (∆T) and can be calculated using the following relation [24]:
It is observed that the Seebeck coefficient displays a positive sign, which indicates that the BFPT samples exhibit p-type conduction. This fact reflects the higher mobility of holes compared to that of electrons. The fraction of the reduced transition metal ion ratio (C; the ratio of the low to high valence state of Fe) could be determined by using the Seebeck coefficient from Heikes et al. equation as given [25]:
where k is the Boltzmann constant and e is the electronic charge.
The heat treatment temperature-dependence of BFPT samples on the Seebeck coefficient (S) and the fraction of the reduced transition metal ion (C) values at 385 K are summarized in Table 3. It is obvious that the S value tends to be highest for the samples having the lowest C, which is consistent with the SPH mechanism [14, 26].
3.5 Electrical conductivity
3.5.1 Conductivity and activation energy
Temperature dependence of dc conductivity (log σ) as a function of heat treatment temperature for the nanostructured BFPT samples is displayed in Fig. 6. As shown, the dc conductivity is directly proportional to the temperature, which denotes semiconductor behavior [27]. From the figure, a linear dependence of temperature on a certain temperature (called Debye temperature θD, obtained in Table 4, defined as the uppermost temperature for a single normal oscillation) θD/2 is observed. Therefore, the data obtained from the measured conductivity above θD/2 were fitted with the SPH model suggested by Austin and Mott [28, 29]. The behavior of dc conductivity can be well described by the Mott formula [15]:
where W denotes the activation energy, k denotes Boltzmann’s constant and T denotes the absolute temperature.
The activation energy (W) was obtained from the slope above a certain temperature (385 K). The pre-exponential factor (σ0) was determined using the intercept of the least square straight-line fitting of the experimental conductivity data as illustrated in Fig. 6. The calculated values of (W) and (σ) for the nanostructured BFPT samples as the effect of heat treatment temperature at 385 K are listed in Table 2. The electrical conductivity of the BFPT samples increases with the effect of heat treatment temperature. As can be seen from the figure, the conductivity of the heat treated sample at 673 K lower than that of the as-milled. This is probably due to the porous character of the pellet, which causes the mean Fe − O − Fe spacing to be larger than that for the as-milled sample and hence makes electronic hopping more difficult.
The effect of heat treatment temperature on dc conductivity (log σ) at 385 K and reduced transition metal ion (C) for nanostructured BFPT samples is shown in Fig. 7. As illustrated from the figure, as the H.T temperature of the measured samples increases, there is also an increase in C (C = Fe+2/∑Fe); the electrical conductivity displays the same behavior. In the present samples, including TMO which is Fe2O3, the electrical conduction has been confirmed to be of an electronic nature [14, 26]. It is expected that the hopping of electrons from a low to a higher valence state (Fe2+ → Fe3+ and vice versa) is responsible for the conduction process in the BFPT samples.
The heat treatment temperature effect on the dc conductivity and activation energy at 385 K for the nanostructured BFPT samples is shown in Fig. 8. It is observed from the figure that, as the magnitude of the conductivity (σ) increases, the activation energy (W) decreases with increasing heat treatment temperature, which is compatible with the SPH mechanism [14, 26].
Austin and Mott have investigated a model for the hopping conductivity in a nanocrystalline semiconductor material [14]. They proposed that the conduction between localized states is owing to SPH [14, 27]. In the non-adiabatic hopping process, the pre-exponential factor σ0 (in Eq. 8) is determined by [30]:
where ν0 denotes the optical phonon frequency (ν0 = kθD/h, h is Planck’s constant), N denotes the transition metal ion density, R denotes interionic distance, C denotes reduced Fe ions fraction (C = Fe+2/∑Fe) and α denotes the wave function decay rate.
Suggesting a strong electron–phonon interaction, Austin and Mott [14] have proposed that:
where WH refers to the polaron hopping energy (WH = WP/2, WP is the binding energy), WD refers to the disorder energy, and is given by [30]:
where εs is static dielectric constant and L represents a constant (L = 0.3).
In the case of adiabatic hopping process, in Eq. 9, when [exp (− 2αR) = 1], therefore σ and σ0 is expressed by [14, 26, 31, 32]:
3.5.2 Small polaron hopping (SPH) parameters
Holstein [33] has investigated a model for the evaluation of polaron hopping energy WH by using the expression [34]:
where \({\left[{\gamma }_{P}\right]}^{2}\) and \({\omega }_{P}\) refer to the electron–phonon coupling constant and the optical phonon frequency, respectively. Bogomolov et al. [35] have estimated the polaron radius (rp) from the mean spacing (R), according to [36]:
The rp values for the nanostructured BFPT are recorded in Table 4. The polaron hopping energy WH could also be calculated by the formula [19]:
Since, the effective dielectric constant (εp) is given by [19]:
where ε∞ and εs point to the dielectric constants of the measured samples at the high frequency and the static frequency, respectively. The range of WH estimated values is (0.268–0.331 eV), which is satisfied for the condition of small polaron formation.
On the basis of Holestein [37], the density of states close to the Fermi level can be described as [31]:
It is observed that the N(EF) values (in Table 4)for the present samples are reasonable for localized states [31, 38].
The electron–phonon interaction values (\({\gamma }_{P}\)) is represented by the following relation [14]:
The estimated values of \({\gamma }_{P}\) are found to be in the range of (5.2–6.5). Mott and Davis [39] have supposed that if the value of \({\gamma }_{P}\) > 4, the electron–phonon interaction for the system is considered to be strong. Therefore, we conclude that the electron–phonon interaction in the nanostructured BFPT samples is strong.
3.5.3 The hopping nature (adiabatic or non-adiabatic)
The nature of the polaron hopping-conduction mechanism (adiabatic or non-adiabatic regime) for the nanostructured BFPT samples can be specified by three methods:
-
(1)
When the pre-exponential factor (σ0), in Eq. 14, is independent of the heat treatment temperature and hardly changes with the sample content, the activation energy (W) should be the main determinant of the conductivity in the adiabatic regime [34, 38]. We computed the factor of σ0 utilizing the experimental values in Table 2. For the nanostructured BFPT, the effect of heat treatment temperature on σ0 is illustrated in Fig. 9. It is observed from the figure that there is almost no change in the σ0 values with the change of the heat treatment temperature. Therefore, σ depends only on W (in Eq. 13) in the adiabatic regime for these samples.
-
(2)
Another method has been proposed by Sayer and Mansingh [32] and Murawski et al. [39]. By plotting the relation of dc conductivity (log σ) against the activation energy (W) at a fixed experimental temperature (Texp) [14, 31] as shown in Fig. 10. If the calculated temperature (Tcal), which is obtained from the slope of the fitting line of such a plot, is close to Texp, then the polaron hopping mechanism nature is the adiabatic regime (i.e., in Eq. 9, exp (− 2αR = 1))[27, 40]. On the other hand, if Tcal is different from Texp, the polaron hopping mechanism nature is the non-adiabatic regime (i.e. in Eq. 9, exp(− 2αR) can’t ignored)) [15, 16]. For the nanostructured BFPT samples, it is noticed that the experimental temperature (Texp = 385 K) is nearly equal to calculated value (Tcal = 378 K) as illustrated in Fig. 10. Therefore, we can be more certain that the conduction mechanism in the BFPT samples is by the adiabatic regime.
-
(3)
Emin and Holestein model [37] suggested a method for determining the nature of the hopping conduction mechanism. In accordance with their model, the polaron band width (J) needs to fulfill the inequality as following [37, 40]:
Since, J can be calculated by [19, 41]:
The values of the right hand side (RHS) in Eq. (21) or (22) at 385K are calculated to be in the order of (0.0136 to 0.145 eV) depending on the heat treatment temperature of the sample. When the values of J are estimated from Eq. 23 as listed in Table 4, it is observed that the RHS values are less than the obtained J values (i.e., Eq. 21 is achieved). Accordingly, we conclude the most suitable nature for the conduction hopping mechanism is in the adiabatic regime.
-
(4)
Emin and Holestein [37] have also proposed the polaron hopping model (supposing WD = 0) based on the model of molecular crystal. Dc conductivity could be obtained by the following [38]:
The resulting values from the experimental data agree with Eq. 24. Therefore, adiabatic hopping is still the predominant process.
We verified that the adiabatic hopping process, in the nanostructured BFPT samples, is the conduction mechanism using the methods discussed above.
3.5.4 The carrier mobility and carrier concentration
The hopping carrier mobility (μ) could be described according to Ausitn and Mott models. As the conduction mechanism was confirmed to be adiabatic, μ can be evaluated by the following expression [27]:
The carrier density (Nc) can be measured using the relation [30]:
The values of µ and Nc of the BFPT samples at a fixed temperature of 385 K are posted in Table 4. According to this table, it was found that μ < \({10}^{-2}\) cm2 V−1 S−1, referring to the fact that the electrons in the BFPT system are localized at the Fe ion sites. Therefore, the system exhibits electron hopping conduction and reconfirms the small polaron hopping formation of this system [27, 38]. Moreover, the values of Nc are approximately constant (Nc 1011 cm−3), indicating that the conductivity in BFPT system is achieved by its hopping mobility [42] (Table 5).
3.5.5 Intermediate variable-range hopping (VRH) model
In accordance with Greaves’ procedure [43], which was based on a modification of Mott’s variable range hopping (VRH) model [31], it could be used at intermediate temperature (below θD/2) and suggested the dc conductivity (σ) by the following equation [27]:
where A and B are constants, B is obtained by [30]:
Based on this procedure and applying Eq. 28, we can plot log (σT1/2) versus T−1/4 as plotted in Fig. 11. It confirms a good fit of the experimental results over the intermediate temperature, indicating that Greave’s VRH model can be applicable for the nanostructured BFPT samples. The evaluated parameters for Greave’s VRH model for nanostructured BFPT samples are given in Table 6. We can calculate the factor α by substituting these data and N(EF) values obtained in Table 4 into Eq. 29. As can be seen, the α and N(EF) values are suitable for the localized states [30, 38].
4 Conclusion
High energy planetary ball mill was performed to prepare 0.52 Bi2O3–0.18 Fe2O3–0.30 PbTiO3 (BFPT) mol% sample. The amorphous phase was attained after milling for 5 h. The milled powder is heat treated at different temperatures of 673, 873, and 1073 K for 5 h. The nanostructure and the formed phases of the BFPT samples were analyzed by XRD and confirmed by HRTEM. The crystal size of the heat-treated BFPT samples increases with increasing the heat-treatment temperature to 873 K; after that, it decreases at 1073 K. The density values follow the same behavior as the crystalline size. According to thermoelectric power properties (TEP), the conduction in the nanostructured BFPT samples is related to mixed charge carriers and the reduced Fe ions fraction. The conductivity behaves similarly to the C fraction (C = Fe+2/∑Fe). The electrical conduction mechanism of these samples in the high-temperature regime is interpreted by the small polaron hopping (SPH) model of Mott–Austin. It was found that Greaves’ variable range hopping (VRH) model was appropriate at intermediate temperatures. It was reported that the small polaron coupling constant was \({\gamma }_{P}\) > 4, indicating a strong electron–phonon interaction. The value of hopping carrier mobility (μ) at 385 K is between 1.99 × 10−3 and 8.65 × 10−3 cm2 V−1 S−1. The density of states at the Fermi level N(EF) is ranged from 0.92 × 1022 to 1.17 × 1022 eV−1 cm−3. The polaron radius (rp) is measured to be 0.177—0.18 nm. The hopping carrier mobility was found to be a fundamental factor in determining the conductivity in the BFPT samples. Studying the conduction mechanism revealed that the nanostructured BFPT samples are consistent with the adiabatic process of SPH model.
References
M.A. Jalil et al., Temperature-dependent phase transition and comparative investigation on enhanced magnetic and optical properties between sillenite and perovskite bismuth ferrite-rGO nanocomposites. J. Appl. Phys. 122(8), 084902 (2017)
D. Maurya et al., BiFeO3 ceramics synthesized by mechanical activation assisted versus conventional solid-state-reaction process: a comparative study. J. Alloy. Compd. 477(1–2), 780–784 (2009)
C. Correas, T. Hungría, A. Castro, Mechanosynthesis of the whole xBiFeO3–(1–x)PbTiO3 multiferroic system: structural characterization and study of phase transitions. J. Mater. Chem. 21(9), 3125–3132 (2011)
Bernardo, M., Synthesis, microstructure and properties of BiFeO. BOLETIN DE LA SOCIEDAD ESPAÑOLA DE, 2014: p. 1.
R. Köferstein, T. Buttlar, S.G. Ebbinghaus, Investigations on Bi25FeO40 powders synthesized by hydrothermal and combustion-like processes. J. Solid State Chem. 217, 50–56 (2014)
H. Jebari et al., Structural, optical, dielectric, and magnetic properties of iron-sillenite Bi25FeO40. Appl. Phys. A 128(9), 842 (2022)
T.L. Burnett et al., Imaging of domains in single crystals of BiFeO3-PbTiO3using various microscopy techniques. J. Phys: Conf. Ser. 26, 239–242 (2006)
M. El-Desoky et al., Transport properties of Ba-doped BiFeO3 multiferroic nanoparticles. J. Mater. Sci: Mater. Electron. 26(9), 6793–6800 (2015)
Z. Ning et al., Achieving both large piezoelectric constant and high Curie temperature in BiFeO3–PbTiO3–BaTiO3 solid solution. J. Eur. Ceram. Soc. 40(6), 2338–2344 (2020)
V.F. Freitas et al., Synthesis and multiferroism in mechanically processed BiFeO3–PbTiO3 ceramics. J. Eur. Ceram. Soc. 31(15), 2965–2973 (2011)
A.E. Hannora, Synthesis of lead–borate glasses using high energy ball milling (attritor). J. Non-Cryst. Solids 429, 1–4 (2015)
J. Benjamin, Mechanical alloying. Sci. Am. 234(5), 40–49 (1976)
S.S. Berbano et al., Formation and structure of Na2S + P2S5 amorphous materials prepared by melt-quenching and mechanical milling. J. Non-Cryst. Solids 358(1), 93–98 (2012)
I.G. Austin, N.F. Mott, Polarons in crystalline and non-crystalline materials. Adv. Phys. 18(71), 41–102 (1969)
A. Hannora, M. El-Desoky, Correlation between Nanostructural and Enhanced Electrical Conductivity of Annealed 30V2O5-20Bi2O3-50P2O5 Glass. Cryst. Res. Technol. 55(10), 2000128 (2020)
A. Al-Syadi et al., Grain size effects on the transport properties of Li3V2 (PO4) 3 glass–ceramic nanocomposites for lithium cathode batteries. J. Mater. Sci: Mater. Electron. 27(4), 4074–4083 (2016)
F. Delogu, G. Cocco, Kinetics of amorphization processes by mechanical alloying: a modeling approach. J. Alloy. Compd. 436(1–2), 233–240 (2007)
K.A. Aly et al., Estimation of lattice strain for zirconia nano-particles based on Williamson-Hall analysis. Mater. Chem. Phys. 193, 182–188 (2017)
S.A. Fareed et al., Structure, Seebeck coefficient and DC electrical conductivity of Bi2Mn4O10 prepared by mechanochemical method. J. Mater. Sci: Mater. Electron. 33(19), 15346–15358 (2022)
M. Baláž et al., Mechanochemistry of copper sulphides: phase interchanges during milling. J. Mater. Sci. 52(20), 11947–11961 (2017)
U.B. Chanshetti et al., Density and molar volume studies of phosphate glasses. Facta Univ.—series: Phys. Chem. Technol. 9(1), 29–36 (2011)
A.E. Harby et al., Correlation between grain size and transport properties of lead titanate based-glass–ceramic nano-composites. J. Mater. Sci.: Mater. Electron. 27(8), 8446–8454 (2016)
M.M. El-Desoky, M.S. Al-Assiri, A.A. Bahgat, Synthesis, structural and transport properties of NaxV2O5⋅nH2O xerogel nanocrystalline thin films. J. Alloy. Compd. 590, 572–578 (2014)
Agarwala, N. International conference on advances in electrical engineering title: Thermoelectric measurements of silicon nanowire arrays for the seebeck effect. in 2013 2nd International Conference on Advances in Electrical Engineering (ICAEE), pp.69–74. IEEE, 2013.
R. Heikes, Thermoelectricity ed RR Heikes and RW Ure (Interscience, New York, 1961), p.81
N.F. Mott, Conduction in non-crystalline materials. Phil. Mag. 19(160), 835–852 (1969)
M.M. El-Desoky, M.M. Abdulrazek, Y.A. Sharaby, Fabrication and electrical properties of reduced graphene oxide doped nanocrystalline vanadium pentoxide films. Mater. Sci. Eng., B 261, 114676 (2020)
A.E. Hannora, M.M. El-Desoky, Effects of heat treatment on the structural and electrical conductivity of Fe2O3–P2O5–PbO glasses. J. Mater. Sci: Mater. Electron. 30(21), 19100–19107 (2019)
A.E. Hannora, M.A. Abbas, M.M. El-Desoky, Annealing effects on the structural, thermal, and electrical properties of 10PbTiO3–10Fe2O3–30V2O5–50B2O3 glass. J. Mater. Sci: Mater. Electron. 32(4), 3998–4007 (2021)
M.M. El-Desoky et al., Impact of sodium oxide, sulfide, and fluoride-doped vanadium phosphate glasses on the thermoelectric power and electrical properties: structure analysis and conduction mechanism. J. Mater. Sci: Mater. Electron. 32(3), 3699–3712 (2021)
N.F. Mott, E.A. Davis, Electronic processes in non-crystalline materials (Oxford University Press, Oxford, 2012)
M. Sayer, A. Mansingh, Transport properties of semiconducting phosphate glasses. Phys. Rev. B 6(12), 4629 (1972)
T. Holstein, Studies of polaron motion: Part II. The “small” polaron. Ann. Phys. 8(3), 343–389 (1959)
M. El-Desoky, Small polaron transport in V2O5–NiO–TeO2 glasses. J. Mater. Sci: Mater. Electron. 14(4), 215–221 (2003)
V. Bogomolov, E. Kudinov, Y.A. Firsov, Polaron nature of current carriers in rutile (TiO2). Sov. Phys. Solid State USSR 9(11), 2502−+ (1968)
S. Yawale, S. Pakade, DC conductivity and hopping mechanism in Bi2O3-B2O3 glasses. J. Mater. Sci. 28(20), 5451–5455 (1993)
D. Emin, T. Holstein, Studies of small-polaron motion IV. Adiabatic theory of the hall effect. Ann. Phys. 53(3), 439–520 (1969)
M.M. El-Desoky, M.S. Al-Assiri, Structural and Polaronic transport properties of semiconducting CuO–V2O5–TeO2 glasses. Mater. Sci. Eng., B 137(1–3), 237–246 (2007)
L. Murawski, C. Chung, J. Mackenzie, Electrical properties of semiconducting oxide glasses. J. Non-Cryst. Solids 32(1–3), 91–104 (1979)
M.M. El-Desoky, DC conductivity and hopping mechanism in V2O5–B2O3–BaO glasses. Phys. Status Solidi (a) 195(2), 422–428 (2003)
M. Al-Assiri, S. Salem, M. El-Desoky, Effect of iron doping on the characterization and transport properties of calcium phosphate glassy semiconductors. J. Phys. Chem. Solids 67(8), 1873–1881 (2006)
M.S. Al-Assiri et al., Structural and transport properties of Li-intercalated vanadium pentoxide nanocrystalline films. Phil. Mag. 90(25), 3421–3439 (2010)
G.N. Greaves, Small polaron conduction IN V2O5-P2O5 glasses. J. Non-crystalline Solids 11, 427446 (1973)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MME-D, NAG, AEH, HAY all author's equal share for contributions.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Desoky, M.M., Gazouly, N.A., Hannor, A.E. et al. Adiabatic small polaron hopping conduction in nanostructured Bi2O3–Fe2O3–PbTiO3 prepared by high energy planetary ball mill. J Mater Sci: Mater Electron 34, 829 (2023). https://doi.org/10.1007/s10854-023-10243-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10243-x