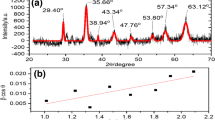
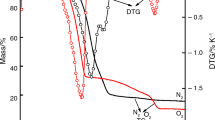
Abstract
The co-precipitation technique was used to successfully produce nanometer cobalt ferrite (CoF). Utilizing the solvent-antisolvent approach, the reduction in the size of sensitive HEM 3-nitro-2,4-dihydro-3H-1,2,4-triazol-5-one (NTO) was effectively achieved. Using simultaneous thermal analysis, the effect of 5% by mass CoF on the thermolysis of NTO and nanosize NTO (r-NTO) was investigated. The kinetic parameter of NTO and r-NTO in the presence of CoF additive was assessed using three isoconversional methods: Flynn–Ozawa–Wall, Kissinger–Akahira–Sunose and Starink. It was found that lowering NTO’s size and adding CoF may both lower the material’s thermal breakdown temperature, with the former dropping it more significantly than the latter. The activation energy of both NTO and r-NTO was raised in the presence of CoF additive.












Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
R.R. Sirach, P.N. Dave, 3-Nitro-1,2,4-triazol-5-one (NTO): high explosive insensitive energetic material. Chem. Heterocycl. Comput. 57, 720–730 (2021). https://doi.org/10.1007/s10593-021-02973-9
S. Hanafi, D. Trache, S. Abdous, Z. Bensalem, A. Mezroua, 5-Nitro-1,2,4-triazole-3-one: a review of recent advance. Chin. J. Energy Mater. 27, 326–347 (2019). https://doi.org/10.11943/CJEM2018371
S. Hanafi, D. Trache, W. He, W.-X. Xie, A. Mezroua, Q.-L. Yan, Catalytic effect of 2D-layered energetic hybrid crystals on the thermal decomposition of 3-nitro-2,4-dihydro-3H-1,2,4-triazol-5-one (NTO). Thermochim. Acta 692, 178747 (2020). https://doi.org/10.1016/j.tca.2020.178747
K.V. Prabhakaran, S.R. Naidu, E.M. Kurian, XRD, spectroscopic and thermal analysis studies on 3-nitro-1,2,4-triazole-5-one (NTO). Thermochim. Acta 241, 199–212 (1994). https://doi.org/10.1016/0040-6031(94)87018-7
M. Zhang, C. Li, H. Gao, W. Fu, Y. Li, L. Tang, Z. Zhou, Promising hydrazinium 3-nitro-1,2,4-triazol-5-one and its analogs. J. Mater. Sci. 51, 10849–10862 (2016). https://doi.org/10.1007/s10853-016-0296-7
D. Kumar, I.P.S. Kapoor, G. Singh, P.F. Siril, A.M. Tripathi, Preparation, characterization, and catalytic activity of nanosized NiO and ZnO: part 74. Propellants Explos. Pyrotech. 36, 268–272 (2011). https://doi.org/10.1002/prep.201000013
G. Yang, F. Nie, J. Li, Q. Guo, Z. Qiao, Preparation and characterization of nano-NTO explosive. J. Energ. Mater. 25, 35–47 (2007). https://doi.org/10.1080/07370650601107104
J.C. Li, Q.J. Jiao, Y.G. Gong, Y.Y. Wang, T. Liang, J. Sun, Explosive performance of HMX/NTO co-crystal. IOP Conf. Ser. Mater. Sci. Eng. 292, 012032 (2018). https://doi.org/10.1088/1757-899X/292/1/012032
C. Guo, H. Zhang, X. Wang, X. Liu, J. Sun, Study on a novel energetic cocrystal of TNT/TNB. J. Mater. Sci. 48, 1351–1357 (2013). https://doi.org/10.1007/s10853-012-6881-5
S. Elbasuney, G.S. El-Sayyad, The potentials of TiO2 nanocatalyst on HMX thermolysis. J. Mater. Sci. Mater. Electron. 31, 14930–14940 (2020). https://doi.org/10.1007/s10854-020-04054-7
S. Elbasuney, A. Hamed, M. Yehia, M. Gobara, M. Mokhtar, The significant impact colloidal nanothermite particles (Fe2O3/Al) on HMX kinetic decomposition. J. Energ. Mater. (2021). https://doi.org/10.1080/07370652.2021.1905107
M. Amiri, K. Eskandari, M. Salavati-Niasari, Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv. Coll. Interface Sci. 271, 101982 (2019). https://doi.org/10.1016/j.cis.2019.07.003
S. Chaturvedi, P.N. Dave, A review on the use of nanometals as catalysts for the thermal decomposition of ammonium perchlorate. J. Saudi Chem. Soc. 17, 135–149 (2013). https://doi.org/10.1016/j.jscs.2011.05.009
S. Chaturvedi, P.N. Dave, N.K. Shah, Applications of nano-catalyst in new era. J. Saudi Chem. Soc. 16, 307–325 (2012). https://doi.org/10.1016/j.jscs.2011.01.015
P.N. Dave, P.N. Ram, S. Chaturvedi, Nanoferrites: catalyst for thermal decomposition of ammonium per chlorate. Part. Sci. Technol. 33, 677–681 (2015). https://doi.org/10.1080/02726351.2015.1023479
A. Miri, M. Sarani, A. Najafidoust, M. Mehrabani, F.A. Zadeh, R.S. Varma, Photocatalytic performance and cytotoxic activity of green-synthesized cobalt ferrite nanoparticles. Mater. Res. Bull. 149, 111706 (2022). https://doi.org/10.1016/j.materresbull.2021.111706
V.R. Bhagwat, A.V. Humbe, S.D. More, K.M. Jadhav, Sol–gel auto combustion synthesis and characterizations of cobalt ferrite nanoparticles: different fuels approach. Mater. Sci. Eng. B 248, 114388 (2019). https://doi.org/10.1016/j.mseb.2019.114388
A.A.H. El-Bassuony, W.M. Gamal, H.K. Abdelsalam, Fascinating study of adding nanocomposite cobalt nano ferrite to silver nanoparticles accompanied magnetite impurity. J. Mater. Sci. Mater. Electron. (2022). https://doi.org/10.1007/s10854-022-08516-y
S. Goktas, A. Goktas, A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: a review. J. Alloys Compd. 863, 158734 (2021). https://doi.org/10.1016/j.jallcom.2021.158734
Y. Lan, J. Zhai, D. Li, R. Yang, The influence of solution chemistry on the morphology of ammonium dinitramide crystals. J. Mater. Sci. 50, 4933–4939 (2015). https://doi.org/10.1007/s10853-015-9040-y
T. Mukundan, G.N. Pur, J.K. Nair, S.M. Pansare, R.K. Sinha, H. Singh, Explosive nitrotriazolone formulates. Def. Sci. J. 52, 127–133 (2002)
A. Saikia, R. Sivabalan, G.M. Gore, A.K. Sikder, Microwave-assisted quick synthesis of some potential high explosives. Propellants Explos. Pyrotech. 37, 540–543 (2012). https://doi.org/10.1002/prep.201100107
Journal of Chemistry: Education Research and Practice | OPAST Online Publishing Group. https://opastonline.com/journal/journal-of-chemistry-education-research-and-practice/current-issue. Accessed 21 Oct 2021
K.Y. Lee, B.W. Asay, J.E. Kennedy, Method for forming energetic nanopowders, U.S. Patent No. 8,557,066. (U.S. Patent and Trademark Office, Washington, DC, 2013)
S. Vyazovkin, A.K. Burnham, J.M. Criado, L.A. Pérez-Maqueda, C. Popescu, N. Sbirrazzuoli, ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 520, 1–19 (2011). https://doi.org/10.1016/j.tca.2011.03.034
J.H. Flynn, L.A. Wall, A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part C Polym. Lett. 4, 323–328 (1966)
T. Ozawa, A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 38, 1881–1886 (1965)
H.E. Kissinger, Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 57, 217–221 (1956)
T. Akahira, T. Sunose, Method of determining activation deterioration constant of electrical insulating materials. Res. Rep. Chiba Inst. Technol. (Sci. Technol.) 16, 22–31 (1971)
M.J. Starink, The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim. Acta 404, 163–176 (2003). https://doi.org/10.1016/S0040-6031(03)00144-8
D. Trache, A. Abdelaziz, B. Siouani, A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Therm. Anal. Calorim. 128, 335–348 (2017). https://doi.org/10.1007/s10973-016-5962-0
S. Jesus Mercy, D. Parajuli, N. Murali, A. Ramakrishna, Y. Ramakrishna, V. Veeraiah, K. Samatha, Microstructural, thermal, electrical and magnetic analysis of Mg2+ substituted cobalt ferrite. Appl. Phys. A 126, 873 (2020). https://doi.org/10.1007/s00339-020-04048-6
J.-T. Wu, J.-G. Zhang, T. Li, Z.-M. Li, T.-L. Zhang, A novel cocrystal explosive NTO/TZTN with good comprehensive properties. RSC Adv. 5, 28354–28359 (2015). https://doi.org/10.1039/C5RA01124H
S. Das, M. Bououdina, C. Manoharan, The influence of cationic surfactant CTAB on optical, dielectric and magnetic properties of cobalt ferrite nanoparticles. Ceram. Int. 46, 11705–11716 (2020). https://doi.org/10.1016/j.ceramint.2020.01.202
F. Mikailzade, H. Türkan, F. Önal, Ö. Karataş, S. Kazan, M. Zarbali, A. Göktaş, A. Tumbul, Structural, optical and magnetic characterization of nanorod-shaped polycrystalline Zn1−xMnxO films synthesized using sol–gel technique. Appl. Phys. A 126, 768 (2020). https://doi.org/10.1007/s00339-020-03953-0
A. Goktas, S. Modanlı, A. Tumbul, A. Kilic, Facile synthesis and characterization of ZnO, ZnO:Co, and ZnO/ZnO: Co nano rod-like homojunction thin films: role of crystallite/grain size and microstrain in photocatalytic performance. J. Alloys Compd. 893, 162334 (2022). https://doi.org/10.1016/j.jallcom.2021.162334
M.A. Ríos-Corripio, B.E. García-Pérez, M.E. Jaramillo-Flores, V.L. Gayou, M. Rojas-López, UV–visible intensity ratio (aggregates/single particles) as a measure to obtain stability of gold nanoparticles conjugated with protein A. J. Nanopart. Res. 15, 1624 (2013). https://doi.org/10.1007/s11051-013-1624-3
H. Kato, A. Nakamura, K. Takahashi, S. Kinugasa, Size effect on UV–Vis absorption properties of colloidal C60 particles in water. Phys. Chem. Chem. Phys. 11, 4946–4948 (2009). https://doi.org/10.1039/B904593G
S. Swathi, R. Yuvakkumar, P.S. Kumar, G. Ravi, D. Velauthapillai, Annealing temperature effect on cobalt ferrite nanoparticles for photocatalytic degradation. Chemosphere 281, 130903 (2021). https://doi.org/10.1016/j.chemosphere.2021.130903
D. Li, H. Song, X. Meng, T. Shen, J. Sun, W. Han, X. Wang, Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials 10, 546 (2020). https://doi.org/10.3390/nano10030546
A. Tumbul, F. Aslan, A. Goktas, M.Z. Zarbali, A. Kilic, Highly stable ethanol-based Cu2ZnSnS4 (CZTS) low-cost thin film absorber: effect of solution aging. Mater. Chem. Phys. 258, 123997 (2021). https://doi.org/10.1016/j.matchemphys.2020.123997
A. Goktas, F. Aslan, I.H. Mutlu, Effect of preparation technique on the selected characteristics of Zn1−xCoxO nanocrystalline thin films deposited by sol–gel and magnetron sputtering. J. Alloys Compd. 615, 765–778 (2014). https://doi.org/10.1016/j.jallcom.2014.06.160
F.R. Mariosi, J. Venturini, V.A. da Cas, C.P. Bergmann, Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications. Ceram. Int. 46, 2772–2779 (2020). https://doi.org/10.1016/j.ceramint.2019.09.266
R.I. Hiyoshi, Y. Kohno, J. Nakamura, Vibrational assignment of energetic material 5-nitro-2,4-dihydro-1,2,4-triazole-3-one (NTO) with labeled isomers. J. Phys. Chem. A 108, 5915–5920 (2004). https://doi.org/10.1021/jp049118i
M. Nazim, A.A.P. Khan, A.M. Asiri, J.H. Kim, Exploring rapid photocatalytic degradation of organic pollutants with porous CuO nanosheets: synthesis, dye removal, and kinetic studies at room temperature. ACS Omega 6, 2601–2612 (2021). https://doi.org/10.1021/acsomega.0c04747
P.M. Kibasomba, S. Dhlamini, M. Maaza, C.-P. Liu, M.M. Rashad, D.A. Rayan, B.W. Mwakikunga, Strain and grain size of TiO2 nanoparticles from TEM, Raman spectroscopy and XRD: the revisiting of the Williamson–Hall plot method. Results Phys. 9, 628–635 (2018). https://doi.org/10.1016/j.rinp.2018.03.008
V. Swamy, B.C. Muddle, Q. Dai, Size-dependent modifications of the Raman spectrum of rutile TiO2. Appl. Phys. Lett. 89, 163118 (2006). https://doi.org/10.1063/1.2364123
Y. Lin, Y.-J. Zhang, W.-M. Yang, J.-C. Dong, F.-R. Fan, Y. Zhao, H. Zhang, N. Bodappa, X.-D. Tian, Z.-L. Yang, G.D. Stucky, Z.-Q. Tian, J.-F. Li, Size and dimension dependent surface-enhanced Raman scattering properties of well-defined Ag nanocubes. Appl. Mater. Today 14, 224–232 (2019). https://doi.org/10.1016/j.apmt.2018.12.012
G. Lan, J. Li, G. Zhang, J. Ruan, Z. Lu, S. Jin, D. Cao, J. Wang, Thermal decomposition mechanism study of 3-nitro-1,2,4-triazol-5-one (NTO): combined TG-FTIR-MS techniques and ReaxFF reactive molecular dynamics simulations. Fuel 295, 120655 (2021). https://doi.org/10.1016/j.fuel.2021.120655
E.F. Rothgery, D.E. Audette, R.C. Wedlich, D.A. Csejka, The study of the thermal decomposition of 3-nitro-1,2,4-triazol-5-one (NTO) by DSC, TGA-MS, and ARC. Thermochim. Acta 185, 235–243 (1991). https://doi.org/10.1016/0040-6031(91)80045-K
R. Dubey, P. Srivastava, I. Kapoor, G. Singh, Synthesis, characterization and catalytic behavior of Cu nanoparticles on the thermal decomposition of AP, HMX, NTO and composite solid propellants, part 83. Thermochim. Acta 549, 102–109 (2012)
V.P. Sinditskii, S.P. Smirnov, VYu. Egorshev, Thermal decomposition of NTO: an explanation of the high activation energy. Propellants Explos. Pyrotech. 32, 277–287 (2007). https://doi.org/10.1002/prep.200700029
Acknowledgements
Author RS is thankful to DST (SR/NM/NT-1014/2016 (G)) for providing Junior Research Fellowship. The authors are grateful to the Department of Chemistry for the research facility, the Department of Physics, Sardar Patel University, India, for providing the XRD and Raman Facility, and Indukaka Ipcowala Center for Interdisciplinary Studies in Science and Technology (IICISST) for Simultaneous thermal analysis.
Funding
The present work was funded by the Department of Science and Technology (DST), Nanomission, New Delhi, India (SR/NM/NT-1014/2016 (G)).
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RS. The first draft of the manuscript was written by RS and PD and both authors commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Informed consent
Not applicable.
Research involving human participants and/or animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dave, P.N., Sirach, R. Investigating the kinetics of the thermolysis of 3-nitro-2,4-dihydro-3H-1,2,4-triazol-5-one (NTO) and reduced size NTO in the presence of cobalt ferrite additive. J Mater Sci: Mater Electron 34, 193 (2023). https://doi.org/10.1007/s10854-022-09643-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09643-2