Abstract
Due to the instability and toxicity issues of lead/tin-based halide perovskites, lead-free metal halide perovskites have emerged as an attractive lead replacement for several semiconductor applications. Here, we present a bismuth (Bi)-based perovskite structure as a low-toxic and potentially substitutable alternative to lead-based perovskite solar cells. The synthesis and optical performance of MAPbI3, MA3Bi2I9Clx, and (MAPbI3:BiCl3) with ratios (of 10, 30, 50, 70)% as lead-free and low lead perovskite are prepared. The grinding technique is used as a green chemistry method compared to a typical reaction for scaling up production. The phase identification, crystallinity, thermal stability, optoelectronic properties, and nanoscale composition are comprised. The results showed that the prepared samples are enhanced in the visible absorption region and aligned well with previous literature. Besides, the bandgap energy for the mixed-structured perovskite, at a molar ratio of 10%, was reduced to 1.52 eV compared to 1.55 and 1.80 eV for MAPbI3, MA3Bi2I9Clx, respectively. At room temperature, the samples emitted intense photoluminescence in the 680–700 nm region. Our findings demonstrate the processability of bismuth perovskites, aiding in the development of high-performance low toxic perovskites by assisting in the refinement of materials and processing methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Throughout the last two decades, several next-generation photovoltaic technologies have attempted to compete with silicon photovoltaics or to fill niche PV applications [1, 2]. The advantage of these photoactive materials is that they are thinner than crystalline silicon and can be sprayed or printed on flexible substrates via low-cost processes [3]. One of the most photoactive technologies is the perovskite solar cell (PSC), invented in 2009 [4, 5]. Organic-inorganic hybrid PSCs have developed significantly since then, with record solar cells currently attaining an outstanding certified 25.7% power conversion efficiency (PCE) [6]. This suggests that perovskites may one day be able to take the place of traditional crystalline silicon solar cells in global markets.
The ideal cubic crystal structure of halide perovskite is ABX3 with a space group \(Pm\stackrel{-}{3}m\) that can achieve band gap tuning by altering all three cation and anion components [7,8,9]. The most widely investigated inorganic-organic halide perovskite unit cell is CH3NH3PbX3 [7, 10,11,12]. Mixed halides perovskites with crystal structures ABX3−xYx and A3B2X9, such as (MAPbI3−xClx, MAPbI3−xBrx, or even MA3Bi2I9, are thought to have high tunable optical qualities, allowing for easy testing with device performance and increasing the overall performance of PSCs [13, 14]. Currently, hybrid organic-inorganic perovskites are the archetypal chemical materials for optoelectronic and high-performance photovoltaic devices [15]. This is owing to several unique merits, including easy and low-cost fabrication methods, long diffusion length, excellent optical absorption with tunable band gaps, flexibility, and transparency [16, 17]. Despite these tremendous achievements, the remaining bottlenecks preventing their large-scale production in the solar markets are their thermal/environmental instability, very short lifetime, and toxicity issues due to lead [18, 19]. Beyond the risks of solar cell production and end-of-life recovery, the possibility of lead leakage induced by encapsulation failures may limit the capacity to employ such cells in real-world applications [20]. To address this, investigation towards lead-free or lead-reduced perovskites has accelerated recently, with tin (Sn) showing reasonable promise among possible alternative metals [21]. The invention of MASnI3 based solar cells with a PCE of more than 6% is one of the most notable achievements [22]. On the other hand, the photovoltaic efficiency of Sn phases in varied Sn/Pb based perovskites has been questioned [23, 24]. Furthermore, Conings have questioned the supposed decreased toxicity of the Sn-based molecule [25, 26]. According to the study, the toxicity of the Sn compound is comparable to that of Pb-based systems. Other problems, such as semiconductor oxidation (unstable in the air because of oxidation of Sn2+ to Sn4+) and device hysteresis, promote the creation of reduced lead perovskites ultimate aim of matching the performance characteristics of lead-based semiconductors [27]. Other work focused on metals that meet the perovskite lattice’s electron valency and size tolerance requirements [28].
Alternatives, toxic-free elements, such as bismuth (Bi), are increasingly extensively regarded as suitable replacements for Pb [28, 29]. As a result, many recent efforts have focused on developing lead-free chemicals, such as Bi-based structures, and their use in functioning optoelectronics. Park et al. [30] proposed bismuth as a lead alternative to limit the usage of Pb. Bi is less hazardous than Pb, and they observed that Cs-based Bi perovskite revealed a PCE of 1.09%; nonetheless, Cs is still well-thought-out hazardous [30, 31]. They also discovered MA3Bi2I9 structure with methylammonium and a PCE of 0.12%, indicating that such a compound performs significantly worse than the archetypal Pb-based MAPbI3 perovskite [32, 33]. Even though Bi-based mixed metal halide composites, their solar cell device efficiency is mediocre, despite their outstanding stability in ambient air [18, 28]. This might be attributable to various causes, including higher exciton binding energy, poor and anisotropic carrier mobility, a wide optical bandgap, and lower absorption. Slavney et al. considered the Cs2AgBiBr6 double-perovskite and discovered that the lattice with 11.25 of the unit-cell axis is crudely double that of MAPbBr3; however, no photovoltaic devices were tested [34]. As a result, looking for different perovskites that are firmer for device synthesis while still functioning in solar cells is intriguing. Other perovskite compounds with photovoltaic characteristics but no lead has been discovered, such as BiMnO3, BiFe2CrO6, and BiFeO3 [35, 36]. Whereas the total PCE of these materials is less minor than that of metal-halide perovskites, they seem more robust and have additional properties. Bi, which is much less poisonous than lead, is used in these cases [37, 38]. As a result, new Bi-based photovoltaic materials with reduced toxicity and environmental effect should be investigated [39]. While Pb-based perovskites have received extensive research, bismuth-doped lead halide perovskites have not been studied in situ, yet no work has been published. As a result, the effect of doping Pb+ 2 with Bi+ 3 on the solidification process of these hybrid metal halides remains unknown. Consequently, this article contributes to a better knowledge of Bi-based metal halide formation by pointing the community toward the most appropriate and effective processing pathways for manufacturing high-quality Bi-based hybrid metal halide perovskites.
To prevent the health concerns associated with toxic lead and toxic solvent inhalation during material fabrication, researchers are investigating Pb-free perovskite materials and toxic-free-solvent techniques [40]. Hence, green chemistry has emerged as widespread knowledge in academia and industry. Green chemistry applies minimization principles to eliminate chemicals and chemical processes [41, 42]. By applying the 12 principles of the green chemistry procedure, the waste materials could be minimized, limiting harmful chemicals, sustaining the atom economy, and protecting the environment, which is our family’s inheritance [43]. This work, which uses green chemistry technology, might help scale up Pb and hazardous solvent-free technology, making it more widely available.
In this work, the mechanochemical solid-state reaction based on grinding methods is utilized to investigate how Bi may be employed as a minimal option to Pb in hybrid perovskite materials. Besides control samples of MAPbI3 and MA3Bi2I9Clx, we have explored a variety of perovskites with the composition of BiCl3 doped MAPbI3 with ratios (10, 30, 50, 70)%. Such specimens were obtained from various precursor materials, including MAI, PbI2, and BiCl3, as well as precursor blend ratios. As a result, our findings shed light on the processability of bismuth-based perovskites, assisting in improving materials and processing techniques for developing high-performance, low-toxic hybrid perovskites.
2 Experimental procedure
2.1 Materials
MAI (≥ 99%, anhydrous, white powder), PbI2 (99.999% trace metals basis, yellow powder), and BiCl3 (99.99% trace metals basis, powder) are obtained from Sigma-Aldrich Company Ltd. All of the compounds utilized without being purified further.
2.2 Synthesis of MAPbI3
The high-purity precursors were weighed into a mortar (made of Zirconia to eliminate precursor contamination effectively) in stoichiometric amounts and ground with a pestle for 50–60 min by hand under an air atmosphere. Consequently, the black-colored MAPbI3 appeared by blending 2.9 g of yellow powder (PbI2) (1 mol) with 1.0 g of (1 mol) MAI white powder, resulting in a total of ~ 3.85 g mixture using a solvent-free solid-state approach until a magnificent structure was obtained. This obtained mixture achieved a percentage yield of 98.6%, implying that only 1.4% of our material was wasted. This could be due to a small amount of sample adhering to the mortar and pestle’s sides. Finally, the ground perovskite powder was transferred to a quartz ampoule and evacuated, ensuring a good vacuum before the ampoule was sealed.
2.3 Synthesis of MA3Bi2I9Clx
The black-colored MA3Bi2I9Clx was prepared by blending 1.98 g of BiCl3 white solid (1 mol) with 1.0 g of MAI white powder (1 mol), yielding a total weight of ~ 2.9 g mixture with a percentage yield of 97.3%.
2.4 Synthesis of BiCl3 doped MAPbI3 with ratios (10, 30, 50, 70)%
Finally, the mixed metal cation-anion powder was made by mixing the precursors of MAI, PbI2, and BiCl3 in different ratios. The MAPbI3:BiCl3 with ratios (90:10)% consisted of 1 g of MAI, 2.61 g of PbI2, and 198.4 mg of BiCl3. The MAPbI3:BiCl3 doped with ratios (70:30)% was made from 1 g of MAI, 2.03 g of PbI2, and 0.595 g of BiCl3. MAPbI3:BiCl3 doped with ratios (50:50)% was made from 1 g of MAI, 1.45 g of PbI2, and 0.992 g of BiCl3. Lastly, MAPbI3:BiCl3 doped with ratios (30:70)% was made from 1 g of MAI, 0.87 g of PbI2, and 1.389 g of BiCl3. All of the above-mentioned prepared mixtures gave a high percentage yield of approximately 97.1%.
2.5 Characterization
The perovskite powders’ crystallographic properties and phase identification were performed using an Empyrean PANalytical X-ray diffractometer (XRD) with a Cu anode X-ray source (Cu Kα1, 1.54060 Å). Raman spectra were collected on a WITec alpha300 instrument using a laser with a 532 nm excitation wavelength at 0.1 mW laser power and an acquisition time of 5 s. The attenuated total reflectance was used to generate Fourier transform infrared (FT-IR) spectra (ATR) configuration (Platinum ATR diamond, Bruker Co.) in the range of 400 to 4000 cm−1. The elemental compositions and valence states of perovskite compositions were determined by using the Thermo Scientific™ K-Alpha™ XPS system. The measurements were conducted using a fully integrated, small-spot size of 400 μm with depth profiling capabilities with a base pressure of 10−9 mbar in the spectrometer and sample handler. The X-ray source is a monochromatic Al-Kα with an excitation energy of 1486.71 eV ranging from − 10 eV to 1350 eV, and a full-spectrum pass energy of 200 eV at a narrow spectrum of 50 eV. A Field-emission scanning electron microscope studied the morphology, structure, and elemental composition (FE-SEM) attached with Energy dispersive X-ray (EDX) analysis (Zeiss, Sigma 500 VP, Germany). The thermal stability of the present compositions was further confirmed by thermogravimetric and differential thermal analyses using a Shimadzu DTG-60 H and Shimadzu TGA-50 H in an interval from 28 to 800 °C at a heating rate of 10 °C min−1 under N2 gas with a flow rate of 30 ml min−1. A UV-Jasco V-570, Japan, coupled in the wavelength range (200–2000) nm within an integrating spherical reflectance unit (ISN), was used to investigate the Abs and R% spectra of several perovskite samples at ambient temperature. A 50 W xenon lamp measured the photoluminescence (PL) spectra at room temperature (Shimadzu RF-5301PC, Kyoto, Japan). All of the data was gathered at room temperature.
3 Results and discussions
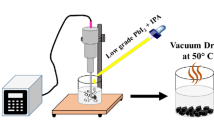
In this work, the Bi-doped Pb halide perovskite was synthesized by CH3NH3I, PbI2, and BiCl3 as starting materials according to the composition BiCl3 doped MAPbI3 with ratios (10, 30, 50, 70)%. In addition, the pure bismuth perovskite is prepared with the formula MA3Bi2I9Clx. The perovskite material was fabricated in this study by employing a solid-state reaction based on grinding techniques. The material generated by grinding is often quick and efficient, yielding the required perovskite phase with no unreacted precursor, as revealed by the XRD profile. In Fig. 1, we present the fabrication of materials via easy green-chemistry-inspired procedures to diminish costs and contrary environmental impact.
3.1 Raman measurements
Raman spectroscopy is a powerful instrument for understanding the vibrational structure of materials such as perovskite materials. Raman spectra of our prepared samples are displayed in Fig. 2a. The Raman spectrum of the MAPbI3 powder obtained by grinding revealed the features of rocking motion, Pb-I-Pb, bending Pb-I stretching, and the multifaceted vibration modes of the CH3NH3 cation. The peaks of the MAPbI3 powder are situated at 71.4, 97.5, and 109 cm−1, as illustrated at the top of Fig. 2a. The MA cations with translations and vibrations are combined to form the low-frequency region’s normal modes at frequencies near 140 cm−1. It can be seen that the MA rotating around the C–N axis is principally responsible for the distinctive characteristic at 130 cm−1 for MAPbI3 structure. This is shifted to higher for MA3Bi2I9Clx perovskite and other MAPbI3 doped by BiCl3 ratios (10, 30, 50, 70)% [44, 45]. Notably, there are shifts in the peaks that happened for the perovskite based on Bi ions, indicating the change in the lattice of the phase transition. According to Ying Lu et al. [46], the Pb-I stretching peaks at 97.5, and 108 cm−1 were attributed to B3g symmetries which mainly represent vibration information about the inorganic components in the material. The translational modes of the organic cation CH3NH3 in MAPbI3 are connected to the peak at 71.4 cm−1. Due to the noticeable vibrations inside the [PbI6]−2 octahedra caused by the phase transition between Pb and Bi, three Raman active vibrating modes are prevalent: I–Pb–Cl asymmetric bending, Bi–Pb–Cl asymmetric stretching, and symmetric Pb–I stretching vibrations [47]. Pb(II) occupies interstitial positions in the MAI crystal lattice and is linearly conjugated to the I-atoms in Bi-doped perovskite. As a result, the interstitial MA3Bi2I9Clx units in the MAX lattice lack such vibrational modes [48].
3.2 FT-IR analysis
FT-IR spectra of the MAPbI3, MA3Bi2I9Clx, and BiCl3-doped MAPbI3 with ratios (10, 30, 50, 70)% perovskites have studied the structure and vibrational peaks of perovskite materials between 400 and 4000 cm−1, as shown in Fig. 2b. The –CH of the alkyl group generally displayed bending at 1250 and 1467 cm−1 for all samples, which is reliable for the CH3 and CH2 groups based on the symmetric bending mode. The asymmetric and symmetric stretching modes of CH2 were also attributed to two significant peaks at 2920 and 2970 cm−1. The C–N bands exhibit stretching vibration between 960 and 971 cm−1 for MAPbI3, MA3Bi2I9Clx, and BiCl3-doped MAPbI3 with ratios (10, 30, 50, 70)% perovskites. The bands at 671 and 906 cm−1 in IR are attributed to CH3 rocking vibration. The NH3+ rocking vibration is located at 1249–1260 cm−1 for all samples. Furthermore, broadband has been seen at 3300–3500 cm−1, linked to the amine groups –NH. The NH3+ bands exhibit asymmetrical stretching vibration at 3170 cm−1 in IR. The bands at 1608 cm−1 are associated with the NH3+ asymmetrical deformation vibration. The bands at 1467 cm−1 and 1465 cm−1 are associated with the NH3+ scissoring vibration. Furthermore, the C–I stretching group was identified by a weak band at 669 cm−1, which was moved to 559 and 591 cm−1 for C–I and C–Cl, respectively [49, 50].
3.3 X-ray diffraction (XRD) analysis
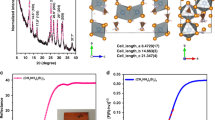
The XRD for pure MAPbI3, MA3Bi2I9Clx, and BiCl3-doping MAPbI3 concentrations with 10%, 30%, 50%, and 70% are displayed in Fig. 3a, b. By investigating the powder XRD data for MAPbI3, the MAPbI3 structure is tetragonal as a symmetry group with I4cm (108) space group, see Fig. S1. The unit cell parameters were specified to be a = b = 8.8718 Å, c = 12.6617 Å [51] after performing Rietveld refinement. The lattice parameters for MAPbI3 are very closely matched with JCPDS card No. 96-451-8044, which confirms high crystallinity and pure perovskite tetragonal. It can be seen that the preferred orientation along the (211) plane appeared, and the signature diffraction intensity peak of PbI2 in the (002) direction at 13.927˚ was observed with the highest peak. For the tetragonal structure, the reflection peaks of (211) and (310) planes were misaligned with the cubic framework, which would be suitable for optimizing the cubic and tetragonal phases [52]. Moreover, the pure bismuth perovskite, MA3Bi2I9Clx, is in good agreement with Park et al. [30] and JCDPS no. 96-711-8492, as illustrated in Fig. 3a. The crystalline phase of this material has already been mentioned in two separate investigations, as reported by Szklarz et al. [53]. The crystal phase Cmcm [63] and an orthorhombic structure were discovered. Nevertheless, Jakubas and Sobczyk planned a hexagonal crystalline structure with the space group P63/mmc(194) comparable to the Cs3Bi2I9 perovskite [54]. The MA3Bi2I9Clx perovskite obtained with this report’s method agrees with the ICSD database for coll. Code 162,078 and the outcomes from Jakubas et al. [54]. In turn, the XRD patterns of the MAPbI3 doped by BiCl3 ratios (10, 30, 50, 70)% are compared in Fig. 3b. The results showed that there are two phases were obtained, as shown in Fig. 3b, (Fig. S2). The main phase is MAPbI3 combined with MA3Bi2Cl9. These data are matched with JCDPS cards no. 96-721-8932 and 96-711-8492 for MAPbI3, and MA3Bi2Cl9, respectively. Finally, from Fig. 3b, all detected peaks are shifted, and the angle shift rises as the quantity of cations increases, indicating that the structure is influenced by cation changes in the perovskite structure, although it may still be near the hexagonal crystalline phase.
3.4 Morphology analysis
It is known that efficient charge extraction will be enhanced by improving the shape that allows charge carriers to be transported efficiently to the electrode. A field-emission scanning electron microscope (FE-SEM) is used to discover the morphology. The MAPbI3, MA3Bi2I9Clx, and BiCl3-doping MAPbI3 concentrations with 10%, 30%, 50%, and 70% perovskite samples were prepared in their powder shape, then their morphology was observed under FE-SEM. Figure 4a, b shows the pure (CH3NH3PbI3) and MA3Bi2I9Clx morphology and represent formed perovskite particles in the homogenous shape, and the particles are regularly distributed in a cubic-like structure. As we can see, small cube-shaped particles are homogeneously distributed. After adding 10% Bi, tiny particles accumulated with enhancement in the grain size of the particles, as shown in Fig. 4c–f. By increasing the doping % of Bi from 30 to 50 to 70%, the shape appears like giant rocks, and some small circular rocks are scattered on it, but the small rocks seem to merge with the large ones, and this can be explained that high doping has integrated with the particles of CH3NH3PbI3 effectively.
The dispersive energy X-ray (EDX) analysis was performed for more investigations. The analysis confirms the elemental compositions and purity of the as-prepared samples, as shown in Table S1 and Fig. S3. The EDS results agree with the molar ratio of the elemental analysis to precisely determine the metal ratios stoichiometry for our prepared materials.
3.5 Thermogravimetric analysis (TGA)
When exposed to heat energy, physical and chemical changes occur in the majority of substances, including organic-inorganic hybrids. These changes are characteristic of such compounds under specific experimental conditions, and it is possibly utilized for qualitative and quantitative examination. The thermal stability of the present MAPbI3 was further confirmed by TGA, as revealed in Fig. 5a. No mass loss was detected when the sample was heated in nitrogen until it reached 338.2 °C. At that point, the composition began to decompose through a weight loss matching to MAI and PbI2. In the second stage, steady weight loss was detected for both compounds up to 600 °C, corresponding to the transition of PbO2 caused by the molecule being degraded in the air. Based on TGA investigations, the following two phases were proposed for the decomposition of MAPbI3. The perovskite atoms begin to separate at approximately 338 °C, with a drop in amine percentages that continues up to 573 °C. As described in the equations below, lead iodide is wholly converted into lead oxide in the second phase [52].
Figure 5b depicts the thermal stability of MA3Bi2I9Clx until 225 °C, after which it loses 93.0% of its initial weight at 340.3 °C. Similarly, Fig. 5c–f shows that loss varies from 280.5 to 422.5 °C depending on the level of Bi-doping (i.e., 10%, 30%, 50%, 70%). Consequently, at 10% doping, there is no mass loss until 324.5 °C before degradation to ≈ 70% of its initial weight at 564.5 °C, as illustrated in Fig. 5c. By increasing the doping ratios by 30%, 50%, and 70%, the thermal stability of the samples is decreased to 458.9 °C, 400.5 °C, and 390.0 °C, respectively, as displayed in Fig. 5d–f.
3.6 Optoelectronic properties
UV-visible spectroscopy is a non-destructive systematic chemistry procedure for determining the quantitative composition of transition metal ions and highly conjugated organic molecules [55]. The optical properties of MAPbI3, MA3Bi2I9Clx, and BiCl3-doping MAPbI3 concentrations with 10%, 30%, 50%, and 70% were determined by measuring the abs and R% spectra of the synthesized powders within 200–1000 nm wavelength. It can be seen that from Fig. 6a, the 10% doped with BiCl3 sample has a higher absorption in the visible region compared to the typical MAPbI3; hence, it appears to be a perfect candidate for solar cell applications. The bandgap energy (Eg) of the as-prepared samples can be calculated via R(%) UV–Vis data using the Kubelka–Munk function as shown from the following formulae [52]:
where R, α, and S denote reflected light, absorption, and scattering coefficients, respectively. The value n equals 1/2 or 2 for direct or indirect band gaps. The calculation procedures were cited in our previous published work [52], as illustrated in Fig. 6b. The band gap values for samples MAPbI3 and MA3Bi2I9Clx, equal 1.547 eV and 1.80 eV, respectively. For BiCl3-doping MAPbI3 concentrations with 10%, 30%, 50%, and 70%, the band gap energies are 1.529 eV,1.536 eV, 2.101 eV, and 1.828 eV, respectively. To compare the optoelectronic data, there is a detailed description of values for absorbance edge, energy gap, PL intensity, and absorbance coefficient of each prepared perovskite, as given in Table 1. As a result, it is possible to state that the energy band gap for Bi-doped with 10% is smaller than others, indicating that it is best suited for solar cell applications.
3.7 Photoluminescence (PL) analysis
In addition to optical characteristics, photoluminescence (PL) spectroscopy was used to identify the features of semiconductor material based on the intrinsic and extrinsic [56]. Figure 7 is illustrated the PL emission spectra of MAPbI3, MA3Bi2I9Clx, and MAPbI3:BiCl3 with ratios of 10%, 30%, 50%, and 70% at roughly 700 nm at room temperature. An excitation band measured the PL spectra (507 nm) [57]. MAPbI3:BiCl3 with ratios 50% jumped at high intensity compared to others in the PL intensity. The non-monotonic behavior of the PL signal of all samples indicates that the observed PL emission is not related to a band-to-band transition, but its origin can be attributed to a color change which can be adequate to the humidity nature and light during measurements. In addition, double perovskite crystals or mixed halides such as MAPbI3:BiCl3 with ratios of 10%, 30%, 50%, and 70% are expected to overcome the limitation of classic hybrid organic-inorganic perovskite crystals related to the presence of lead and the lack of structural stability. Perovskites are ionic crystals in which the carriers are expected to be strongly coupled to lattice vibrations. In this work, the PL emission in MAPbI3:BiCl3 is strongly influenced by the strong electron-phonon coupling. This can be understood as a competition between recombination pathways; the carriers can either be trapped by the color centers or relax to the minimum of the indirect band gap [58, 59]. Because of the effective charge transmission produced through the doping action of ingredients, this astounding PL was accepted. The existence of PL quenching in our compounds specified that (i) the creation of excitons is dependent on photoabsorption efficacy; (ii) charge splitting can arise at an edge owing to excitons’ migration average; and (iii) the power of charge splitting can arise at an interface.
3.8 X-ray photoelectron spectroscopy (XPS)
In this article, XPS measurements were employed on only pure MAPbI3 and MAPbI3-doped with 10% BiCl3 perovskite samples to investigate the interaction of organic-inorganic species. In addition, the element composition after precisely small incorporation of BiCl3 material and understanding the impact of BiCl3 on the bulk effect of environmental factors on both materials and surface stability of MAPbI3 is confirmed. Figure 8a shows the broad range XPS survey spectrum of a typical MAPbI3 perovskite, which reveals peaks at binding energies of 402.49 eV and 284.80 eV, respectively, matching the photoelectron peaks of N 1s and C 1s. In addition, the doublet peaks of I and Pb are found, with concomitant spin-orbit splitting, at 619.29 eV and 138.45 eV, respectively. Consequently, this broad energy spectrum reveals the presence of C, N, Pb, and I components in the produced powder, consistent with prior findings [60], illustrating the purity and complete conversion of MAPbI3 perovskite.
However, on the other hand, in the survey spectrum of the 10% doped BiCl3, the structure is elucidated by the elements including C, N, Pb, I, and Bi. According to the intensity reliance of core levels, the I 4d and Pb 5d core levels may precisely detect the I/Pb proportions in the sample: they are the same kind of orbital. Because their binding energies are identical, the observed electrons come from the same probing depth. Unfortunately, to check whether the chloride sits on the crystal surface, as was the case for other detection methods, the amount of chloride was below the detection limit of the XPS instrument. Table 2 shows the main quantification results, including atomic concentrations of the core levels extracted from XPS survey spectra of MAPbI3 and MAPbI3-doped with 10% BiCl3 samples. After Shirley-type noise removal, the XPS spectra were discretized with the CasaXPS program that used a non-linear least-squares fitting approach. The surface atomic (%) was determined using the manufacturer’s sensitivity factors from the relevant peak regions. The narrow scan XPS spectra of the I 3d, Bi 4f, and Pb 4f regions for MAPbI3 and MAPbI3-doped with 10% BiCl3 are described in Fig. 8a–d. MAPbI3’s deconvoluted XPS spectra of the core level I 3d doublets of 3d3/2 and 3d5/2 at 619.29 and 630.76 eV correspond to the I3 charge are illustrated in Fig. 8b. According to previous studies [61], the lower binding component of I 3d situated at 619.29 eV is referred to as triiodide I3− and the existence of oxidized iodine at the top of the surface, which generates the iodate cation with the iodate anion (IO2), might be indicated by the extra widening signal at 622.3 eV. The characteristic peak separation of the associated spin-orbit splitting is determined at 4.88 eV. Correspondingly, for MAPbI3-doped with 10% BiCl3, the exact Fig. 8b shows the XPS spectra of I 3d, where two peaks were observed at 619.5 and 630.95 eV, which are characteristic of 3d5/2 and 3d3/2, respectively, indicating the existence of I in the (− 1) state. Accordingly, an insignificant shift of ∼ 0.2 eV to advanced binding energies is detected for the I 3d spectrum in MAPbI3-doped with 10% BiCl3, which might correspond to a relatively higher oxidation state or effect of the local chemical environment.
Furthermore, for MAPbI3-doped with 10% BiCl3, the Bi 4f binding energy region was also measured in detail, and the results are presented in Fig. 8c. The presence of Bi 4f core level was found in the region of 165.0 to 157 eV, and it can be separated into four peak positions: (157.23, 159.21) for 4f7/2 and (162.55 and 164.6 eV) for 4f5/2, indicating there were two states of Bi in the MAPbI3-doped with 10% BiCl3 [62, 63]. One was associated with 10% BiCl3 binding energy (157.23 eV and 162.55 eV), and the other to surface Bi3+ ions coordinated with Cl− (159.21 and 164.6 eV), indicating the Bi present in the Bi3+ oxidation state. These obtained data are almost consistent with Meiying Leng et al. [64].
Finally, for Pb 4f core level, in the case of MAPbI3, there are two intense peaks located at 138.45 eV and 143.33 eV, corresponding to 4f7/2 and 4f5/2, respectively, with a distinct peak separation of 4.88 eV, indicating the presence of Pb+ 2 that could be ascribed to the Pb–I bonds in perovskite [65], as shown in Fig. 8d. At lower binding energies, the prepared material exhibits two additional peaks reflecting the presence of metallic Pb(0), frequently detected in the XPS spectra of halide perovskites [64]. Similarly, for a perovskite with mixed ions of the MAPbI3-doped with 10% BiCl3, the Pb 4f spectrum slightly shifts positively to up 0.28 eV with higher binding energies.
4 Conclusion
To summarize, the synthesis of lead-free perovskite materials had an essential impact on the efficacy of the optoelectronic application. The utilization of the solid-state grinding procedure is regarded as a novel and promising green chemical strategy for producing perovskite materials. Interstitial Bi(III) doping into the Pb-X matrix was proposed new material without using toxic solvents. The final products showed the highest percentage yield of 97–98.6%, indicating that the reactions are completely efficient with negligible loss. We achieved excellent crystallinity and purity with this strategy giving rapid, efficient, and providing the required perovskite structure, as evidenced by XRD, SEM-EDX, FTIR, XPS, and Raman characterizations. The prepared samples were enhanced in the visible region and aligned well with previously reported results. Furthermore, with a molar ratio of 10%, the bandgap energy for the mixed-structured perovskite was reduced to 1.52 eV, compared to 1.55 and 1.80 eV for MAPbl3 and MA3Bi2l9Clx, respectively. The samples emitted intense photoluminescence at room temperature in the 680–700 nm range. Thermogravimetric analysis proved the thermal stability of the prepared samples within the range from 225 to 460 °C, depending on the doping rate. Finally, the absorbance of light by a perovskite layer generated with the grinding method demonstrated a high crystallinity and an excellent microstructure.
Data availability
Some or all data of this study are available from the corresponding author upon reasonable request.
References
G. Giuliano, A. Bonasera, G. Arrabito, B. Pignataro, Semitransparent perovskite solar cells for building integration and tandem photovoltaics: design strategies and challenges. Solar Rrl. 5, 2102100702 (2021)
S. Ghosh, R. Yadav, Future of photovoltaic technologies: a comprehensive review. Sustain Energy Technol. 47, 101101410 (2021)
M.A. Almheiri, PhD, University of Cambridge, Lucy Cavendish College (2018)
D.B. Liu et al., Energy level bending of organic-inorganic halide perovskite by interfacial dipole. Phys. Status Solidi (RRL)–Rapid Res. Lett. 13, 1900103 (2019)
C.Y. Xu et al., Coordinated optical matching of a texture interface made from demixing blended polymers for high-performance inverted perovskite solar cells. ACS Nano. 14, 196–203 (2019)
https://www.nrel.gov/pv/cell-efficiency.html. (2021)
Y. Zhang et al, Ambient fabrication of Organic-Inorganic hybrid Perovskite Solar cells. Small Methods 5, e2000744 (2021)
R.K. Ulaganathan et al., Stable formamidinium-based centimeter long two-dimensional lead halide perovskite single-crystal for long-live optoelectronic applications. Adv. Funct. Mater. 32(15), 2112277 (2022)
M.S. Selim, A.M. Elseman, Z. Hao, ZnO nanorods: an advanced cathode buffer layer for inverted perovskite solar cells. ACS Appl. Energy Mater. 3, 11781–11791 (2020)
A.K. Jena, A. Kulkarni, T. Miyasaka, Halide perovskite photovoltaics: background, status, and future prospects. Chem. Rev. 119, 3036–3103 (2019)
F. Robles Montes et al., Study of DMSO concentration on the optical and structural properties of perovskite CH3NH3PbI3 and its use in solar cells. J. Solid State Chem. 312, 123158 (2022)
A.M. Elseman, M.M. Rashad, Influence of nitrogen atmosphere one-step heating assisted the solution processing of Kesterite Cu2ZnSnS4 as hole extraction on the efficacy of the inverted perovskite solar cells. Opt. Mater. 124, 111998 (2022)
Y. Wang et al., Reliable measurement of perovskite solar cells. Adv. Mater. 31, e1803231 (2019)
N. Calisi et al., Thin films deposition of fully inorganic metal halide perovskites: a review. Mater. Sci. Semicond. Process. 147, 106721 (2022)
L. Jonathan et al., Hybrid organic-inorganic perovskite halide materials for photovoltaics towards their commercialization. Polymers 14, 1059 (2022)
A.E. Shalan, S. Kazim, S. Ahmad, Lead-free perovskites: metals substitution towards environmentally benign solar cell fabrication. ChemSusChem 12, 4116–4139 (2019)
N.-G. Park, M. Grätzel, T. Miyasaka, K. Zhu, K. Emery, Towards stable and commercially available perovskite solar cells. Nat. Energy 1, 1–8 (2016)
I. Mesquita, L. Andrade, A. Mendes, Perovskite solar cells: materials, configurations and stability. Renew. Sustain. Energy Rev 82, 2471–2489 (2018)
H.J. Snaith, S. Lilliu, The path to Perovskite on Silicon PV. Sci. Video Protocols 1, 1–8 (2018)
Y.I. Lee et al., A low-temperature thin-film encapsulation for enhanced stability of a highly efficient perovskite solar cell. Adv. Energy Mater. 8, 1701928 (2018)
G. Schileo, G. Grancini, Lead or no lead? Availability, toxicity, sustainability and environmental impact of lead-free perovskite solar cells. J. Mater. Chem. C 9, 67–76 (2021)
E.S. Parrott et al., Effect of structural phase transition on charge-carrier lifetimes and defects in CH3NH3SnI3 perovskite. J. Phys. Chem. Lett. 7, 1321–1326 (2016)
F. Gu et al., Lead-free tin‐based perovskite solar cells: strategies toward high performance. Solar Rrl 3, 1900213 (2019)
P. Wu, S. Wang, X. Li, F. Zhang, Beyond efficiency fever: preventing lead leakage for perovskite solar cells. Matter 5, 1137–1161 (2022)
M.-C. Tang et al., Bismuth-Based Perovskite‐Inspired solar cells: in situ diagnostics reveal similarities and differences in the Film formation of Bismuth‐and lead‐based Films. Solar RRL 3, 1800305 (2019)
A. Babayigit et al., Assessing the toxicity of Pb-and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 6, 1–11 (2016)
K.J. Savill, A.M. Ulatowski, L.M. Herz, Optoelectronic properties of tin–lead halide perovskites. ACS Energy Lett. 6, 2413–2426 (2021)
S. Attique et al, A potential checkmate to lead: bismuth in organometal halide perovskites, structure, properties, and applications. Adv. Sci. 7, 1903143 (2020)
T. Atsue, I.B. Ogunniranye, O.E. Oyewande, Investigation of material properties of halide mixed lead - free double perovskite for optoelectronic applications using first-principles study. Mater. Sci. Semicond. Process. 133, 105963 (2021)
B.W. Park et al, Bismuth based hybrid perovskites A3Bi2I9 (A: methylammonium or cesium) for solar cell application. Adv. Mater. 27, 6806–6813 (2015)
N.C. Miller, M. Bernechea, Research update: bismuth based materials for photovoltaics. APL Mater. 6, 084503 (2018)
F. Ünlü et al, Single-or double A-site cations in A3Bi2I9 bismuth perovskites: what is the suitable choice? J. Mater. Res 36, 1794–1804 (2021)
S.M. Masawa, J. Li, C. Zhao, X. Liu, J. Yao, 0D/2D mixed dimensional lead-free caesium bismuth iodide perovskite for solar cell application. Materials 15, 2180 (2022)
A.H. Slavney, T. Hu, A.M. Lindenberg, H.I. Karunadasa, A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J. Am. Chem. Soc. 138, 2138–2141 (2016)
R. Nie et al., Mixed sulfur and iodide-based lead-free perovskite solar cells. J. Am. Chem. Soc. 140, 872–875 (2018)
R. Nechache et al, Bandgap tuning of multiferroic oxide solar cells. Nat. Photonics 9, 61–67 (2015)
H. Suzuki et al., Organobismuth chemistry (Elsevier, Amsterdam, 2001)
H. Needleman, Lead poisoning. Annu. Rev. Med. 55, 209–222 (2004)
M.S. Shadabroo, H. Abdizadeh, M.R. Golobostanfard, Elpasolite structures based on A2AgBiX6 (A: MA, cs, X: I, Br): application in double perovskite solar cells. Mater. Sci. Semicond. Process. 125, 105639 (2021)
S.M. Jain, T. Edvinsson, J.R. Durrant, Green fabrication of stable lead-free bismuth based perovskite solar cells using a non-toxic solvent. Commun. Chem. 2, 1–7 (2019)
O.V. Kharissova, B.I. Kharisov, C.M. Oliva González, Y.P. Méndez, I. López, Greener synthesis of chemical compounds and materials. Royal Soc. Open Sci. 6, 191378 (2019)
M. Baláž, P. Baláž, M.J. Sayagués, A. Zorkovská, Bio-inspired mechanochemical synthesis of semiconductor nanomaterial using eggshell membrane. Mater. Sci. Semicond. Process. 16, 1899–1903 (2013)
B.I. Kharisov, O.V. Kharissova, Handbook of greener synthesis of nanomaterials and compounds. Synthesis at the macroscale and nanoscale (CRC Press, Boca Raton, 2021)
F. Brivio et al., Lattice dynamics and vibrational spectra of the orthorhombic, tetragonal, and cubic phases of methylammonium lead iodide. Phys. Rev. B 92, 144308 (2015)
M.A. Pérez-Osorio et al., Vibrational properties of the organic–inorganic halide perovskite CH3NH3PbI3 from theory and experiment: factor group analysis, first-principles calculations, and low-temperature infrared spectra. J. Phys. Chem. C 119, 25703–25718 (2015)
Y. Lu et al., in Journal of Physics: Conference Series. (IOP Publishing, 2020), vol. 1637, pp. 012080
A. Kaltzoglou et al., Optical-vibrational properties of the Cs2SnX6 (X = cl, br, I) defect perovskites and hole-transport efficiency in dye-sensitized solar cells. J. Phys. Chem. C 120, 11777–11785 (2016)
S. Das Adhikari et al., Continuous-flow synthesis of orange emitting sn (II)‐doped CsBr materials. Adv. Opt. Mater. 9, 2101024 (2021)
H.-P. Boehm, H. Knözinger, In Catalysis (Springer, Berlin, 1983), pp.39–207
M.M. Rashad, A.M. Elseman, A.M. Hassan, Facile synthesis, characterization and structural evolution of nanorods single-crystalline (C4H9NH3) 2PbI2X2 mixed halide organometal perovskite for solar cell application. Optik 127, 9775–9787 (2016)
J. Ding et al., High-quality inorganic–organic perovskite CH3NH3PbI3 single crystals for photo-detector applications. J. Mater. Sci 52, 276–284 (2017)
A.M. Elseman, M.M. Rashad, A.M. Hassan, Easily attainable, efficient solar cell with mass yield of nanorod single-crystalline organo-metal halide perovskite based on a ball milling technique. ACS Sustain. Chem. Eng. 4, 4875–4886 (2016)
P. Szklarz et al., Structure, phase transitions and molecular dynamics of [C (NH2) 3] 3 [M2I9], M = sb, Bi. J. Phys.: Condens. Matter. 20, 255221 (2008)
R. Jakubas, L. Sobczyk, Phase transitions in alkylammonium halogenoantimonates and bismuthates. Phase Transitions: A Multinational Journal 20, 163–193 (1990)
H. Jiang et al., Non-destructive detection of multi-component heavy metals in corn oil using nano-modified colorimetric sensor combined with near-infrared spectroscopy. Food Control 133, 108640 (2022)
T.P. van der Pol, K. Datta, M.M. Wienk, Janssen, the intrinsic photoluminescence spectrum of perovskite films. Adv. Opt. Mater. 10, 2102557 (2022)
M. Mączka et al, [Methylhydrazinium] 2PbBr4, a ferroelectric hybrid Organic–Inorganic Perovskite with multiple nonlinear optical outputs. Chem. Mater 33, 2331–2342 (2021)
H. Li et al., Unusual pressure-driven phase transformation and band renormalization in 2D vdW hybrid lead halide perovskites. Adv. Mater. 32, 1907364 (2020)
E. Kirstein et al., Lead-dominated hyperfine interaction impacting the carrier spin dynamics in halide perovskites. Adv. Mater. 34, 2105263 (2022)
T. Ye, National University of Singapore (Singapore), (2017)
B.-M. Bresolin, C. Günnemann, D.W. Bahnemann, M. Sillanpää, Pb-free Cs3Bi2I9 perovskite as a visible-light-active photocatalyst for organic pollutant degradation. Nanomaterials 10, 763 (2020)
M. Leng et al., Surface passivation of bismuth-based perovskite variant quantum dots to achieve efficient blue emission. Nano Lett. 18, 6076–6083 (2018)
B.-M. Bresolin et al, Anchoring lead-free halide Cs3Bi2I9 perovskite on UV100–TiO2 for enhanced photocatalytic performance. Sol. Energy Mater. Sol. Cells 204, 110214 (2020)
F.C. Lang, Stability and properties of methylammonium lead iodide solar cells. (2017)
I.M. Asuo et al., All-ambient‐processed CuSCN as an inexpensive alternative to spiro‐OMeTAD for perovskite‐based devices. Energy Technol. 9, 2000791 (2021)
Acknowledgements
This work is supported by the Academy of Scientific Research & Technology (ASRT), Grant No. 6462, Science Up program, Egypt. In addition, the first author thanks the national institute of standards (NIS-Egypt) for continuous support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MRAE: Conceptualization, Methodology, Writing-Original Draft. AME: Supervision, Methodology and Suggested experimental advice. AAA: Supervision, Editing. HMH: Supervision. AH: Supervision and Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsayed, M.R.A., Elseman, A.M., Abdelmageed, A.A. et al. Green and cost-effective morter grinding synthesis of bismuth-doped halide perovskites as efficient absorber materials. J Mater Sci: Mater Electron 34, 194 (2023). https://doi.org/10.1007/s10854-022-09574-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09574-y