Abstract
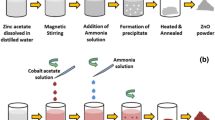
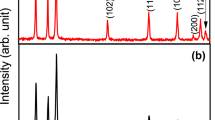
Present study portrays, physicochemical investigations of pristine and Pd2+ modified ZnO nanoflowers (NFs) compositional series [Zn(1−x)PdxO NFs; where x = 0, 0.01, 0.03, 0.05, and 0.07] synthesized via sol–gel reaction route. X-ray diffractogram of all the compositions displayed hexagonal wurtzite type crystallinity with P63mc space group without any traces of impurities as confirmed from Rietveld refinement technique. It was found that the average crystallite size increased from 32 to 74 nm with Pd2+ intrusion in the crystal lattice framework of ZnO. FESEM micrographs depicted that the surface morphology of pure and Pd modified compositions exhibit flower-shaped surface morphology. The optical properties of as-synthesized NFs were carried out using UV–vis spectroscopy and it was observed that optical energy bandgap (Eg) decreased minutely by Pd2+ doping. To determine the gas sensing behavior of synthesized NFs, the prepared gel during material fabrication was used to cast thin films. The thin films were calcined at 600 °C for LPG gas sensing activity and it was observed that the transient curve shows a high response for the composition x = 0.03 for the concentration of 2000 ppm. The present result illustrates that synthesized NFs showed potential candidature for LPG gas sensing. The present study reveals synthesized NFs showed potential candidature for LPG gas sensing 80 °C with a gas sensitivity of 315%.











Similar content being viewed by others
Data availability
The data was acquired through experimentation, and the institution provided other material.
References
A. Sutti, C. Baratto, G. Calestani, C. Dionigi, M. Ferroni, G. Faglia, G. Sberveglieri, Inverse opal gas sensors: Zn(II)-doped tin dioxide systems for low-temperature detection of pollutant gases. Sens. Actuators B 130(1), 567–573 (2008). https://doi.org/10.1016/j.snb.2007.11.048
D.S. Dhawale, D.P. Dubal, A.M. More, T.P. Gujar, C.D. Lokhande, Room temperature liquefied petroleum gas (LPG) sensor. Sens. Actuators B 147(2), 488–494 (2010). https://doi.org/10.1016/j.snb.2010.02.063
G. Singh, R.C. Singh, Highly sensitive and selective liquefied petroleum gas sensor based on novel ZnO–NiO heterostructures. J. Mater. Sci.: Mater. Electron. 30(22), 20010–20018 (2019). https://doi.org/10.1007/s10854-019-02368-9
K. Hanabusa, S. Takata, M. Fujisaki, Y. Nomura, M. Suzuki, Fluorescent gelators for detection of explosives. Bull. Chem. Soc. Jpn. 89(11), 1391–1401 (2016). https://doi.org/10.1246/bcsj.20160232
G. Lawrence et al., A nanoporous cytochrome c film with highly ordered porous structure for sensing of toxic vapors. Adv. Mater. 29(42), 1–7 (2017). https://doi.org/10.1002/adma.201702295
S. Yang, C. Jiang, S. Huai Wei, Gas sensing in 2D materials. Appl. Phys. Rev. (2017). https://doi.org/10.1063/1.4983310
I. Osica et al., Highly networked capsular silica-porphyrin hybrid nanostructures as efficient materials for acetone vapor sensing. ACS Appl. Mater. Interfaces 9(11), 9945–9954 (2017). https://doi.org/10.1021/acsami.6b15680
C. Wang, L. Yin, L. Zhang, D. Xiang, R. Gao, Metal oxide gas sensors: sensitivity and influencing factors. Sensors 10(3), 2088–2106 (2010). https://doi.org/10.3390/s100302088
S. Das, V. Jayaraman, SnO2: a comprehensive review on structures and gas sensors. Prog. Mater. Sci. 66, 112–255 (2014). https://doi.org/10.1016/j.pmatsci.2014.06.003
S.A. Vanalakar et al., Controlled growth of ZnO nanorod arrays via wet chemical route for NO2 gas sensor applications. Sens. Actuators B 221(2), 1195–1201 (2015). https://doi.org/10.1016/j.snb.2015.07.084
S.P. Patil et al., Spray pyrolyzed indium oxide thick films as NO2 gas sensor. Ceram. Int. (2016). https://doi.org/10.1016/j.ceramint.2016.07.135
S.B. Jagadale, V.L. Patil, S.A. Vanalakar, P.S. Patil, Preparation, characterization of 1D ZnO nanorods and their gas sensing properties. Ceram. Int. (2017). https://doi.org/10.1016/j.ceramint.2017.11.116
S.S. Shendage et al., Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens. Actuators B 240(2), 426–433 (2017). https://doi.org/10.1016/j.snb.2016.08.177
H. Nanto, T. Minami, S. Takata, Zinc-oxide thin-film ammonia gas sensors with high sensitivity and excellent selectivity. J. Appl. Phys. 60(2), 482–484 (1986). https://doi.org/10.1063/1.337435
B. Karunagaran, P. Uthirakumar, S.J. Chung, S. Velumani, E.K. Suh, TiO2 thin film gas sensor for monitoring ammonia. Mater. Charact. (2007). https://doi.org/10.1016/j.matchar.2006.11.007
B. Wang, L.F. Zhu, Y.H. Yang, N.S. Xu, G.W. Yang, Fabrication of a SnO2 nanowire gas sensor and sensor performance for hydrogen. J. Phys. Chem. C 112(17), 6643–6647 (2008). https://doi.org/10.1021/jp8003147
T. Sen, N.G. Shimpi, S. Mishra, R. Sharma, Polyaniline/γ-Fe2O3 nanocomposite for room temperature LPG sensing. Sens. Actuators B 190, 120–126 (2014). https://doi.org/10.1016/j.snb.2013.07.091
S. Maiti, S. Pal, K.K. Chattopadhyay, Recent advances in low temperature, solution nanoarchitectures for electron emission and. CrystEngComm 17, 9264–9295 (2017). https://doi.org/10.1039/C5CE01130B.This
Ü. Özgür et al., A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98(4), 1–103 (2005). https://doi.org/10.1063/1.1992666
C.S. Rout, A.R. Raju, A. Govindaraj, C.N.R. Rao, Hydrogen sensors based on ZnO nanoparticles. Solid State Commun. 138(3), 136–138 (2006). https://doi.org/10.1016/j.ssc.2006.02.016
S.C. Ko, Y.C. Kim, S.S. Lee, S.H. Choi, S.R. Kim, Micromachined piezoelectric membrane acoustic device. Sens. Actuators A 103(1–2), 130–134 (2003). https://doi.org/10.1016/S0924-4247(02)00310-2
D.P. Hansora, N.G. Shimpi, S. Mishra, Performance of hybrid nanostructured conductive cotton materials as wearable devices: an overview of materials, fabrication, properties and applications. RSC Adv. 5(130), 107716–107770 (2015). https://doi.org/10.1039/c5ra16478h
Q. Wan et al., Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 84(18), 3654–3656 (2004). https://doi.org/10.1063/1.1738932
W.T. Moon, Y.K. Jun, H.S. Kim, W.S. Kim, S.H. Hong, CO gas sensing properties in Pd-added ZnO sensors. J. Electroceram. 23(2–4), 196–199 (2009). https://doi.org/10.1007/s10832-007-9377-y
M.J.S. Spencer, I. Yarovsky, ZnO nanostructures for gas sensing: interaction of NO2, NO, O, and N with the ZnO(\(10\overline{10}\)) surface. J. Phys. Chem. C 114(24), 10881–10893 (2010). https://doi.org/10.1021/jp1016938
S. Xu, Z.L. Wang, One-dimensional ZnO nanostructures: solution growth and functional properties. Nano Res. 4(11), 1013–1098 (2011). https://doi.org/10.1007/s12274-011-0160-7
T. Trindade, J.D. Pedrosa De Jesus, P. O’Brien, Preparation of zinc oxide and zinc sulfide powders by controlled precipitation from aqueous solution. J. Mater. Chem. 4(10), 1611–1617 (1994). https://doi.org/10.1039/jm9940401611
L. Guo, Y.L. Ji, H. Xu, P. Simon, Z. Wu, Regularly shaped, single-crystalline ZnO nanorods with wurtzite structure. J. Am. Chem. Soc. 124(50), 14864–14865 (2002). https://doi.org/10.1021/ja027947g
B. Weintraub, Z. Zhou, Y. Li, Y. Deng, Solution synthesis of one-dimensional ZnO nanomaterials and their applications. Nanoscale 2(9), 1573–1587 (2010). https://doi.org/10.1039/c0nr00047g
J. Zhang, L. Sun, J. Yin, H. Su, C. Liao, C. Yan, Control of ZnO morphology via a simple solution route. Chem. Mater. 14(10), 4172–4177 (2002). https://doi.org/10.1021/cm020077h
G. Korotcenkov, The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R 61(1–6), 1–39 (2008). https://doi.org/10.1016/j.mser.2008.02.001
N.G. Shimpi, S. Jain, N. Karmakar, A. Shah, D.C. Kothari, S. Mishra, Synthesis of ZnO nanopencils using wet chemical method and its investigation as LPG sensor. Appl. Surf. Sci. 390, 17–24 (2016). https://doi.org/10.1016/j.apsusc.2016.08.050
H. Li, S. Jiao, S. Gao, H. Li, L. Li, Dynamically controlled synthesis of different ZnO nanostructures by a surfactant-free hydrothermal method. CrystEngComm 16(38), 9069–9074 (2014). https://doi.org/10.1039/c4ce01282h
E. Oh et al., High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation. Sens. Actuators B 141(1), 239–243 (2009). https://doi.org/10.1016/j.snb.2009.06.031
M.W. Ahn, K.S. Park, J.H. Heo, D.W. Kim, K.J. Choi, J.G. Park, On-chip fabrication of ZnO-nanowire gas sensor with high gas sensitivity. Sens. Actuators B 138(1), 168–173 (2009). https://doi.org/10.1016/j.snb.2009.02.008
S.D. Lee, S.H. Nam, M.H. Kim, J.H. Boo, Synthesis and photocatalytic property of ZnO nanoparticles prepared by spray-pyrolysis method. Phys. Procedia 32, 320–326 (2012). https://doi.org/10.1016/j.phpro.2012.03.563
M. Dhingra, N.K. Singh, S. Shrivastava, P.S. Kumar, S. Annapoorni, Worm like zinc oxide nanostructures as efficient LPG sensors. Sens. Actuators A 190, 168–175 (2013). https://doi.org/10.1016/j.sna.2012.11.015
D.B. Bharti, A.V. Bharati, Synthesis of ZnO nanoparticles using a hydrothermal method and a study its optical activity. Luminescence 32(3), 317–320 (2017). https://doi.org/10.1002/bio.3180
J.A. Alvarado, A. Maldonado, H. Juarez, M. Pacio, Synthesis of colloidal ZnO nanoparticles and deposit of thin films by spin coating technique. J. Nanomater. (2013). https://doi.org/10.1155/2013/903191
N. Salah et al., High-energy ball milling technique for ZnO nanoparticles as antibacterial material. Int. J. Nanomed. 6, 863–869 (2011). https://doi.org/10.2147/ijn.s18267
P.C. Chang et al., ZnO nanowires synthesized by vapor trapping CVD method. Chem. Mater. 16(24), 5133–5137 (2004). https://doi.org/10.1021/cm049182c
P.P. Sahay, R.K. Nath, Al-doped zinc oxide thin films for liquid petroleum gas (LPG) sensors. Sens. Actuators B 133(1), 222–227 (2008). https://doi.org/10.1016/j.snb.2008.02.014
N. Kakati, S.H. Jee, S.H. Kim, J.Y. Oh, Y.S. Yoon, Thickness dependency of sol–gel derived ZnO thin films on gas sensing behaviors. Thin Solid Films 519(1), 494–498 (2010). https://doi.org/10.1016/j.tsf.2010.08.005
A. Dey, S. Roy, S.K. Sarkar, Synthesis, fabrication and characterization of ZnO-based thin films prepared by sol-gel process and H2 gas sensing performance. J. Mater. Eng. Perform. 27(6), 2701–2707 (2018). https://doi.org/10.1007/s11665-018-3284-z
A. Gupta, S. Rohilla, Synthesis, characterization and Rietveld refinement of AgBr@KNi [FeC6N6]0.7[CoC6N6]0.3/SiO2 nanocomposite. Mater. Today 44, 4282–4286 (2020). https://doi.org/10.1016/j.matpr.2020.10.545
R. Wahab et al., Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater. Res. Bull. 42(9), 1640–1648 (2007). https://doi.org/10.1016/j.materresbull.2006.11.035
E.P. Etape, J. Foba-Tendo, L.J. Ngolui, B.V. Namondo, F.C. Yollande, M.B.N. Nguimezong, Structural characterization and magnetic properties of undoped and Ti-doped ZnO nanoparticles prepared by modified oxalate route. J. Nanomater. (2018). https://doi.org/10.1155/2018/9072325
A.A. Joraid, A.S. Solieman, M.A. Al-Maghrabi, M.H. Almutairy, Studies of crystallization kinetics and optical properties of ZnO films prepared by sol–gel technique. J. Sol–Gel Sci. Technol. 97(3), 523–539 (2021). https://doi.org/10.1007/s10971-020-05467-w
R. Selvanayaki et al., Structural, optical and electrical conductivity studies of pure and Fe doped zinc oxide (ZnO) nanoparticles. Mater. Today 49, 2628–2631 (2022). https://doi.org/10.1016/j.matpr.2021.08.045
S.K. Abdel-Aal, A.S. Abdel-Rahman, Graphene influence on the structure, magnetic, and optical properties of rare-earth perovskite. J. Nanopart. Res. 22(9), 1–10 (2020)
S.K. Abdel-Aal, A.I. Beskrovnyi, A.M. Ionov, R.N. Mozhchil, A.S. Abdel-Rahman, structure investigation by neutron diffraction and x-ray diffraction of graphene nanocomposite CuO–rGO prepared by low-cost method. Physica Status Solidi (a) 218(12), 2100138 (2021)
S.K. Abdel-Aal, M.F. Kandeel, A.F. El-Sherif, A.S. Abdel-Rahman, Synthesis, characterization, and optical properties of new organic–inorganic hybrid perovskites [(NH3)2(CH2)3]CuCl4 and [(NH3)2(CH2)4]CuCl2Br 2. Physica Status Solidi (a) 218(12), 2100036 (2021)
X.J. Wang, W. Wang, Y.L. Liu, Enhanced acetone sensing performance of Au nanoparticles functionalized flower-like ZnO. Sens. Actuators B 168, 39–45 (2012). https://doi.org/10.1016/j.snb.2012.01.006
R. Wahab, F. Khan, Y.K. Mishra, J. Musarrat, A.A. Al-Khedhairy, Antibacterial studies and statistical design set data of quasi zinc oxide nanostructures. RSC Adv. 6(38), 32328–32339 (2016). https://doi.org/10.1039/c6ra05297e
Y.H. Elbashar, A.E. Omran, J.A. Khaliel, A.S. Abdel-Rahaman, H.H. Hassan, Ultraviolet transmitting glass matrix for low power laser lens. Nonlinear Opt. Quantum. Opt.: Mod. Opt. 49, 247–265 (2018)
Y.H. Elbashar, A.E. Omran, J.A. Khaliel, A.S. Abdel-Rahaman, H.H. Hassan, Ultraviolet transmitting glass matrix for low power laser lens. Nonlinear Opt. Quantum. Opt.: Mod. Opt. 51, 171–193 (2018)
Y.H. Elbashar, A.E. Omran, J.A. Khaliel, A.S. Abdel-Rahaman, H.H. Hassan, Ultraviolet transmitting glass matrix for low power laser lens. Nonlinear Opt. Quantum. Opt.: Mod. Opt. 51, 195–212 (2018)
S.K. Abdel-Aal, A.S. Abdel-Rahman, Fascinating physical properties of 2d hybrid perovskite [(NH3)(CH2)7(NH3)] CuClxBr4−x, x= 0, 2 and 4. J. Electron. Mater. 48(3), 1686–1693 (2019)
U.T. Nakate, R.N. Bulakhe, C.D. Lokhande, S.N. Kale, Au sensitized ZnO nanorods for enhanced liquefied petroleum gas sensing properties. Appl. Surf. Sci. 371, 224–230 (2016). https://doi.org/10.1016/j.apsusc.2016.02.196
M.S. Al-Assiri, M.M. Mostafa, M.A. Ali, M.M. El-Desoky, Structural and gas sensing properties of annealed ZnO thin film. Silicon 8(3), 361–367 (2016). https://doi.org/10.1007/s12633-015-9390-8
S. Choudhary, S. Annapoorni, R. Malik, Evolution and growth mechanism of hexagonal ZnO nanorods and their LPG sensing response at low operating temperature. Sens. Actuators A 293, 207–214 (2019). https://doi.org/10.1016/j.sna.2019.04.048
M. Tonezzer, T.T. Le Dang, N. Bazzanella, V.H. Nguyen, S. Iannotta, Comparative gas-sensing performance of 1D and 2D ZnO nanostructures. Sens. Actuators B 220, 1152–1160 (2015). https://doi.org/10.1016/j.snb.2015.06.103
A.K. Singh, Microwave assisted growth of ZnO nanorods and nanopolypods nanostructure thin films for gas and explosives sensing. J. Nanopart. 2013, 1–12 (2013). https://doi.org/10.1155/2013/783691
M.N. Cardoza-Contreras, J.M. Romo-Herrera, L.A. Ríos, R. García-Gutiérrez, T.A. Zepeda, O.E. Contreras, Single ZnO nanowire-based gas sensors to detect low concentrations of hydrogen. Sensors (Switzerland) 15(12), 30539–30544 (2015). https://doi.org/10.3390/s151229816
V.R. Shinde, T.P. Gujar, C.D. Lokhande, R.S. Mane, S.H. Han, Use of chemically synthesized ZnO thin film as a liquefied petroleum gas sensor. Mater. Sci. Eng. B 137(1–3), 119–125 (2007). https://doi.org/10.1016/j.mseb.2006.11.008
S. Bhatia, N. Verma, R.K. Bedi, Ethanol gas sensor based upon ZnO nanoparticles prepared by different techniques. Results Phys. 7, 801–806 (2017). https://doi.org/10.1016/j.rinp.2017.02.008
S. Mondal, S. Bhattacharya, P. Mitra, Structural, morphological, and LPG sensing properties of Al-doped ZnO thin film prepared by SILAR. Adv. Mater. Sci. Eng. (2013). https://doi.org/10.1155/2013/382380
A. Yadav, B.C. Yadav, A mechanochemical synthesis of nanostructured zinc oxide via acetate route for LPG sensing. J. Exp. Nanosci. 9(5), 501–511 (2014). https://doi.org/10.1080/17458080.2012.671541
K.V. Gurav, P.R. Deshmukh, C.D. Lokhande, LPG sensing properties of Pd-sensitized vertically aligned ZnO nanorods. Sens. Actuators B 151(2), 365–369 (2011). https://doi.org/10.1016/j.snb.2010.08.012
Acknowledgements
The authors are grateful to the EMDL Department of Physics and Astrophysics, University of Delhi, permission for work in the laboratory, and Chandigarh University for the XRD and FESEM facility.
Author information
Authors and Affiliations
Contributions
PP: conceptualization, investigation. MD: supervision. NK: review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participate
Informed consent was obtained from all authors included in the study.
Consent to publish
Consent was obtained from all authors to publish in the journal.
Research involving human and/or animal participants
This chapter does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patial, P., Deshwal, M. & Kumar, N. Physicochemical investigations of Pd2+ substituted ZnO nanoflowers for liquefied petroleum gas sensing. J Mater Sci: Mater Electron 33, 11768–11782 (2022). https://doi.org/10.1007/s10854-022-08141-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08141-9