Abstract
Transparent and electrical conducting p-type off-stoichiometric copper–chromium oxide thin films were used to build p-Cu0.66Cr1.33O2/n-ZnO heterojunctions. The junctions were fabricated in a novel and simple five step process including metal organic chemical vapour deposition, atomic layer deposition, chemical wet etching, and optical lithography. One last step of thermal annealing, with varying temperatures of 650 and 700 °C, is added in order to tune the electrical properties of delafossite and consequently the electrical features of p–n junctions. This work was developed to address the lack of transparent and industrially scalable rectifying p–n junctions that can open multiple application paths in transparent electronics. A competitive ideality factor η of 6.6 and a transmittance in the visible range of 50% were achieved. An understanding of the electronic response of junctions is presented herein as well as a deepening comprehension of the physical properties of materials, with the bands alignment and the Fermi level tuning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the recent years, wide bandgap semiconductors have been investigated in order to build a transparent p–n junction. Challenges still exist in creating a heterojunction that combines a relevant bands alignment with a satisfying rectifying behaviour while remaining optically transparent over the visible wavelength range [1]. Simple transparent p–n junctions can derive into photodetectors and photovoltaic cells and can open paths to more complex device as transparent transistors [2]. From this perspective, transparent conductive oxides (TCOs) are a special kind of materials that combine high electrical conductivity, interesting carrier mobility and large optical bandgap [3, 4]. Homo-junctions were explored in the past, mainly focused on the zinc oxide [2]. Despite that its p-type conductivity was reported (under non-equilibrium conditions) [5], the problem of dopants asymmetry inducing furthermore the lack of reliability of the p-type layer impedes an adequate utilization of ZnO homo-junctions. The best electrical performances, combining high ION/IOFF ratio and ideality factor closer to 1 were reported by Grundmann et al. [2] for NiO/ZnO (ION/IOFF = 2 × 1010 at ± 2 V and an ideality factor η = 1.9) and for CuI/ZnO (ION/IOFF = 6 × 106 at ± 2 V and an ideality factor η = 2.1). The ratio between the diode forward current and the off-state current, measured at a given ± V, is a standard measurement of the quality of the diode, by the distinction of a clear ON and OFF state. The ideality factor is a factor that relates how close the diode behaves compared with the ideal equation, which is a factor related to the carriers recombination, where 1, represents normally a diode limited by the minority carriers. However the measured transmittance was under 50% for both cases, a value not well fitted for transparency. Moreover, the fabrication method was pulsed laser deposition (PLD), a high-cost technique with important limitations in terms of industrial uses, since it is nearly impossible to prepare uniform thin films on a large scale area [6].
Among n-type TCOs, indium tin oxide (ITO) is already widely used in flat panel displays, touch screens or light emitting diodes. Nonetheless the high cost of indium and its toxicity reclaim alternative solutions and at the moment the most promising one seems to be the zinc oxide (ZnO), a direct bandgap semiconductor with suitable electrical and optical properties (Eg = 3.4 eV [7], transmittance above 80% in the visible region [8,9,10]), high chemical resistance and low fabrication cost compared with ITO. ZnO, in its un-doped or doped forms (Al:ZnO or Ga:ZnO) starts already to find their way into applications [11,12,13,14].
Whilst the problem of finding an adequate n-type semiconductor seems to be solvable/solved, the search for a matching p-type semiconductor is still ongoing. The challenge in achieving a capable p-type TCO rise from the intrinsic electronic structure of metal oxides with the top of the valence band composed by strongly localized oxygen 2p orbitals. This results furthermore in difficulties in introducing shallow acceptors and consequently high effective masses of carriers [13]. In 1997, Kawazoe et al. [14], suggested a new method for the modulation of the valence band for AMO2 (with A = Cu or Ag and M = Al, Sc, Cr, Fe, Ga, or Y) [15] with the delafossite structure. This modulation is explained by the covalent bonding between Cu 3d10 and O 2p6 (with close energy levels), resulting in a dispersion of the top valence band that furthermore ameliorates holes delocalisation. This model explains thus the higher holes mobility (above 1 cm2 V−1 s−1) achieved in this type of materials when comparing with other TCOs [18]. Among copper delafossites, CuCrO2 presents a high density of states of 3d cations (Cr3+) near the valence band maximum allowing thus the covalent mixing between the chromium and oxygen atoms and a good dopability [17]. The interest in these particular materials has grown since Nagarajan et al. [19] reported the highest conductivity (220 S cm−1) of delafossite compounds for Mg doped CuCrO2 whilst they measured an optical transparency of 30% in the case of 270 nm thin films. There are usually two ways of doping CuCrO2 thin films, intrinsic doping which is attributed to copper vacancies and oxygen interstitials, and extrinsic doping of which Mg is the most common, but also Fe, N or Zn can be considered [13]. Recent papers report the fabrication of a new peculiar phase of copper chromium delafossite characterized by an important deficit of copper. High electric conductivities and adequate transparency in visible range [16] were measured for such thin films, deposited using chemical vapour deposition.
With the controlled tunability of carrier concentration and good increase of optical transmittance over the annealing processes [17] this particular material might became a viable option for the introduction and study of a transparent p–n junction [18]. As-deposited films present high carrier concentration, up to 1022 cm−3, attributed to chains of missing copper [19]. Although of great interest for p-type TCO applications, these high values are unsuitable for building reliable p–n junctions. Fortunately, the metastable nature of these peculiar defects makes possible the tuning of carrier concentration by using thermal annealing steps. During such processes the copper chains vacancies are progressively healed while the films stoichiometry and morphology remain unaltered. By using annealing temperatures within 650–900 °C range the carrier concentration was diminished from 1021 to 1017 cm−3 while the electrical conductivity and drift mobility were varied few orders of magnitude. More important, the manipulation of Fermi level and the improvement of optical transmittance from 38% to values above 60% were reported [17].
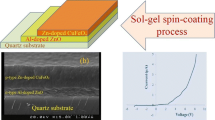
Several works reporting previously the fabrication of CuCrO2/ZnO p–n junctions were using only PLD [20,21,22,23] as the fabrication method. As already mentioned, this fabrication method is not suitable for industrial applications due to its lack of efficient scalability. The present paper suggests a new versatile and technological readable approach combining MOCVD, ALD, lithography followed by an annealing step in order to create a better interface and to produce a more favourable band-alignment. This process, schematically illustrated in Fig. 1 provides a promising, simple, straightforward and cheap fabrication method, easily scalable into large area for industrial purposes (Fig. 1). The junction was annealed at 650 °C and at 700 °C. The electrical and optical behaviour of the junction is therefore compared for these annealing temperatures, together with a deeper investigation of the changes in the physical properties of materials.
2 Materials and methods
Cu0.66Cr1.33O2 films were deposited on a sapphire substrate using Direct Liquid Injection—Metal Organic Chemical Vapour Deposition system DLI-MOCVD, (MC200 from Annealsys). The used copper and chromium precursors are bis[2,2,6,6-tetramethyl-3,5-heptanedionato] copper(II) and tris[2,2,6,6- tetramethyl-3,5-heptanedionato]chromium(III), respectively (Cu(thd)2 and Cr(thd)3, Strem Chemicals). Cyclohexane solutions with a total precursor concentration of [Cu(thd)2] = [Cr(thd)3] = 2,5 mM were used. The total canister solution used was 1 L of Cyclohexane with 1.075 g Cu(thd) + 1.5 g Cr(thd). The deposition parameters used were: substrate temperature of 450 °C, oxygen flow of 2000 sccm, nitrogen flow of 850 sccm and the total process pressure was 12 mbar. The process used followed the previous optimization detailed by Lunca Popa et al. [18]. ALD deposition of ZnO was next deposited, on glass substrate for the thin film study and on top of the delafossite film, deposited on sapphire to make a p–n junction, following the process described by Roge et al. [24] at 150 °C. Argon was used as purge and carrier gas, for 500 cycles and with a pulse sequence: Diethylzinc (DEZ, Zn(C2H5)2) pulse 150 ms—purge 10 s—H2O pulse 200 ms—purge 10 s.
The annealing processes were performed at 650 and 700 °C in a Rapid Thermal Annealing reactor (from Annealsys) at a pressure of 0.3 mbar under a nitrogen flow of 25 sccm. The temperature step for heating and cooling was 5 °C s−1. The annealing time considered includes only the plateau regime. Prior to deposition, the substrates were cleaned in acetone and ethanol and dried with nitrogen jet.
Kevin Probe Force Microscopy (KPFM) measurements have been performed on a Bruker Innova using the surface potential mode as amplitude modulation. Surface topography is obtained in the first pass and the surface potential is measured on the second pass. Freshly cleaved highly-oriented pyrolitic graphite (HOPG) is used as reference. The measurements are performed under dry N2 atmosphere to avoid contaminations on the surface.
The films morphology and thickness were inspected using scanning electron microscopy (SEM) using 10 kV of acceleration voltage. The structure of the grown films was studied by X-Ray Diffraction (XRD) using a Bruker D8 Discover system using monochromatic Cu Kα radiation (l = 1.54 Å) at 40 kV and 40 mA. The scans were performed in the grazing incidence (GI) geometry using a scanning step of 0.02°.
The patterning processes were performed by means of lithography. First a layer of HDMSwas spin-coated at 4000 rpm for 30 s, with an with an acceleration of 1000 rpm s−2. It was then baked for 60 s at 115 °C. Then a layer of Shipley S1813 was spin-coated for 60 s at 4000 rpm, with an acceleration of 1000 rpm s−2, leading to a 1.2 µm layer. After it was baked for 60 s at 115 °C. The exposition was made with a MLA 150 from Heidelberg Instruments using a dose of 90 mJ cm−2. After exposure, the samples were developed using MF319 for 40 s and rinsed for 30 s. The samples were dipped in FeCl2 at 0.1% for 15 s to etch the ZnO, with an expected etched thickness of 100 nm.
The electrical characterization of the junction used a configuration with two points at different voltage, with a voltage sweep and measuring the current between the two tungsten tips. The sweep tip was kept on the Cu0.66Cr1.33O2 contacted with silver paste and constant 0 V was kept on ZnO pads. The carrier concentration of ZnO was measured using Hall-effect measurements with a magnet of 0.562 T using Van der Pauw configuration. In the case of the delafossite, these values were extracted from the measurement of the Seebeck coefficient, using small polaron model.
3 Results and discussion
The fabrication of transparent diodes based on ZnO and Cu0.66Cr1.33O2, started with the deposition of Cu0.66Cr1.33O2 on sapphire substrate at 450 °C followed by ZnO deposited at 150 °C. Sapphire was chosen as a substrate due to its high temperature stability being adequate for wide temperature range studies.
The thickness of films as measured by cross-sectional SEM, was found to be 250 nm for Cu0.66Cr1.33O2. The zinc oxide layer was measured by elipsometry on a silicon sample located next to the sapphire sample during the ALD deposition, resulting in 65 nm as shown in Fig. 2. As deposited ZnO presents a carrier concentration of 5 × 1019 cm−3, with an associated conductivity of 120 S cm−1. While delafossite films present a carrier concentration of 2 × 1021 cm−3, with an associated conductivity of 20 S cm−1. Films present high electrical conductivities, that leads to an ohmic behaviour of the junction, showing a non-rectifying response as later described. The annealing effects on the delafossite have been reported in our previous work [17]. A decrease of the carrier concentration for temperatures above 650 °C was observed. With 700 °C annealing for 15 min, a decrease of the carrier concentration to 1019 cm−3 is obtained, for the delafossite films. On the other hand annealing ZnO at 650 °C for 15 min has also a significant impact reducing the carrier concentration to values below 1018 cm−3.
Figure 3 depicts a projection of the band-diagram of the films, using the combination of data from Refs. [25,26,27] and the results of measurements of the work function for the different annealing temperatures. The reported electron affinities for ZnO and CuCrO2 suggests a type-II band alignment in between our delafossite and ZnO.
Projection of the band-diagram using the combination of data from Refs. [25,26,27], where was extracted the electronic affinity, with the measurements of the work function for the different annealing temperatures (as-deposited, after 650 °C annealing and after 700 °C annealing) performed by KPFM, presenting a type-II band alignment in between our delafossite and ZnO
KPFM measurements were performed on samples, corresponding to the different annealing temperatures presented herein, as-deposited, 650 °C and 700 °C. The measurements were performed alternatively between HOPG reference and the sample, in order to compensate for drifts due to contaminations of the tip. In order to compensate the vacuum levels misalignment KPFM insert a voltage VDC = (Φtip − Φsample)/e where, Φtip (Pt–Ir) = 5.5 eV. The work function ΔΦ is measured according to:
where χ is the electronic affinity (2.1 eV for copper chromium delafossite [27], 4.3 eV for zinc oxide [25, 26]), Eg is the band gap (3.2 eV for copper chromium delafossite [27], 3.4 eV for zinc oxide [25, 26]). We assume the these values (Χ,Eg) are insignificantly affected by annealing [19, 28] and thus holds for both cases of annealed and as-deposited.
In Fig. 3 is shown the change of the Fermi level as extracted from KPFM measurements upon annealing. In both films, the Fermi level shifts towards midgap position after annealing. This might be correlated with the decrease in carrier concentration, as previously described. This prooves the controllable tuning of the Fermi level with the annealing steps.
Furthermore the optical transmittance dependence on the annealing conditions is represented in Fig. 4, for individual films. An increase in optical transmittance from 38 up to 50% is measured for the case of Cu0.66Cr1.33O2 whilst no major difference was observed in the case of ZnO after the annealing step. Cu0.66Cr1.33O2 and ZnO transmittance measurements were performed on sapphire and glass substrates, respectively. The optical properties of the junction were investigated for the junction, deposited in sapphire, annealed at 700 °C in between 400 and 800 nm resulting in an optical transmittance of 48%, which could be further increased using another substrate (moving from sapphire to glass).
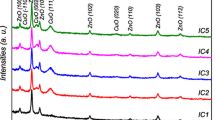
Fig. 5 shows the grazing incidence X-Ray diffractograms in the interest range of films (a) delafossite deposited on sapphire substrate, (b) ZnO deposited on glass substrate, (c) final junction, with stacked ZnO and delafossite on a sapphire substrate, after a thermal annealing step at 700 °C. The diffraction peak of delafossite can be clearly attributed to the (012) diffraction plan, (2θ = 36.4°), with a seemingly preferential orientation, as previously discussed [19]. ZnO diffraction patterns exhibited three distinct peaks, that might be associated to (100), (002) and (101) planes according to pdf card 04-006-2557, (2θ = 31.8°, 34.4° and 36.3°, respectively). Regarding the diode sample, the representative peaks of delafossite and ZnO thin films are observed, with a major response to the delafossite (012) plane. There is a seemingly preferential orientation of the ZnO deposited on delafossite as compared with ZnO thin films deposited on glass, leading to a relative bigger contribution of the (002) peak as compared with the (100). The (101) peak might be hidden within more prominent in the delafossite (012) peak.
The chemical composition of the junction as a function of depth was analysed using Secondary-Ion Mass Spectrometry (SIMS), as represented in Fig. 6. Layers are chemically stable with no inter-layer diffusion.
The optical transmittance of the junction was measured in the optical range 400–800 nm after annealing at 700 °C for 15 m, with a resulting transmittance of 50%, for the bilayer deposited on sapphire substrates.
Electrical properties of the junction were then studied, with a focus on the ratio of forward to reverse current, ION/IOFF, calculated at ± 4 V.
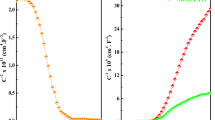
Prior to annealing, no rectifying behaviour is presented by the junction. After the first annealing at 650 °C, a rectifying diode behaviour, with ION/IOFF = 7, was measured. Another thermal treatment at 700 °C was subsequently done improving furthermore the electrical results. Room temperature I–V characteristics of the junction (a) without thermal annealing, (b) with thermal annealing at 650 °C and (c) with thermal annealing at 700 °C are presented in Fig. 7.
Current–voltage characteristics of p- Cu0.66Cr1.33O2/n-ZnO diode with, (a) no thermal treatment, (b) annealed at 650 °C, (c) annealed at 700 °C, comparing with an ideal diode fitting, Eq. (2) for (b and c). Inset semi log current–voltage characteristics of the p–n junction annealed at 700 °C used for extraction of the ideality factor
The best ION/IOFF = 13 was achieved after the annealing at 700 °C. The increase was mainly due to the reduction of IOFF, which may be attributed to the reduction of interface traps and recombination centres [23]. However, there is a trade-off with the increase in performance due to the better junction quality with the reduced forward current with higher annealing temperature, since the turn-on voltage of the device increases, as we extract hereafter.
The I–V curve of the junction after the thermal treatment at 700 °C, is also presented in Fig. 7, with a fitting as of an ideal diode, following the Eq. (2):
where, Vt = kT/e; η is the diode ideality factor; V is the applied voltage and is considered I1 ≠ I2 due to a shunt resistance effect for small voltages, I2 being the reverse saturation current. The reverse current was extracted, IOFF = − 15 nA, as the current at − 4 V.
For V > 4 V, the linear response of the curve is governed by the junction series resistance, allowing to extract the series resistance value with a linear I–V fitting between 4 < V < 5. The extracted value is Rs = 2 MΩ, a quite large value. Comparing Eq. (2), with the commonly used diode Eq. (3), it was extracted Rsh = 1.8 kΩ.
The turn-on voltage (Von) of the heterojunction, was estimated to be 1.4 V for the junction annealed at 700 °C and 0.9 V for the 650 °C. The shift of the I–V curves is due to the reduction of recombination centres at the interface [23]. The I0, presented in Eq. 3, was extracted with the value 16 nA. The ideality factor of the junction was calculated using a linear fit in the semi-log current vs voltage characteristic of the junction in between 0.2 < V < 0.6, resulting in η = 6.3 (Fig. 7 inset). These high ideality factor values are commonly reported in the case of wide gap heterojunctions and values even higher were reported [2, 21].
According to Shah et al. [29], the ideality factor is the sum of the several ideality factors present in the many local rectifying junctions of the device and thus values > 2 are expected. Zekry et al. [30, 31], claim that this value can also be justified by the recombination of carriers at the interface of the heterojunction, as well as the effect provided by the metal-semiconductor contacts. This might be non-ohmic and have a relatively high resistance that can impact the extracted ideality factor. The films roughness and the different crystalline orientations introducing mismatches, defects and inhomogeneities at the interface can also result in η > 2 [32].
A type-II band diagram of a p–n junction is exhibited in Fig. 8. The workfunction of both sides of the junction after annealing at 700 °C were considered. One should notice that the diagram represents a formation of an ideal junction, neglecting defects at the interface. An increase of the ZnO workfunction and a decrease in the case of Cu0.66Cr1.33O2 compared with the values of the films as-deposited, are reported.
Band diagram schematic according to data from literature and workfunction calculated from KPFM measurements, ΔEc represents the energy difference of conduction bands for delafossite—zinc oxide, being 2.2 eV, while ΔEv is the analogous for the valence band difference, being 2.4 eV; EF represents the Fermi level calculated, while it is presented in case of delafossite the difference EF–Ev representing the difference between the Fermi level and the Valence Band with a value of 0.5 eV for the junction annealed at 700 °C and in case of ZnO the difference Ec–EF, representing the difference of the Conduction band and the Fermi level with a value of 1.1 eV for 700 °C annealing
|qΔEc| = 2.2 V, what in a perfect diode would roughly correspond to the turn-on voltage of the diode. The calculated η = 6.3 ideality factor suggests a high interface defect density or may be attributed to the presence of chromium excess for delafossite thin films inducing midgap impurity levels. Therefore a value smaller than the expected 2.2 V is enough to flatten the bands and allow conduction that justifies the smaller turn-on voltage than the one theoretically expected.
4 Conclusion
A transparent p–n junction was created based on highly conductive p-type Cu–Cr–O and n-type ZnO thin films deposited on a sapphire substrate. The electrical behaviour of the junction depends on the post-annealing temperature due to the reported tuning of the Fermi level and carrier concentrations tuning during such thermal treatment. A junction with a rectifying behaviour and an optical transmittance of 50% was finally developed. The ideality factor achieved was η ~ 6, still needs to be improved when compared to the best values reported in literature, of η ~ 2, for junctions with similar transmittance values. The chemical composition of the junction remains unchanged with high temperature annealing steps up to 700 °C. An increase in optical transmittance can be expected by using a more transparent substrate, such as glass, keeping the process safely below 600 °C. The junction was developed as within a 5-step process including techniques easily scalable for a large area deposition, unlike the best performing junctions in the literature, which make recourse to PLD techniques. A higher rectification factor, ION/I OFF, should be optimized engineering a lower series resistance and a better interface engineering may lead to even more performant junction and lower ideality factor.
In conclusion, a simple and scalable process for fabricate a p-Cu0.66Cr1.33O2/n-ZnO transparent junction was demonstrate. This might be of interest in electronic applications based on the principle of transparent diodes.
References
A. Facchetti, T.J. Marks (eds.), Transparent Electronics: From Synthesis to Applications (2010). https://doi.org/10.1002/9780470710609
M. Grundmann, F. Klüpfel, R. Karsthof, P. Schlupp, F.-L. Schein, D. Splith, C. Yang, S. Bitter, H. von Wenckstern, J. Phys. D Appl. Phys. 49, 213001 (2016)
E. Muchuweni, T.S. Sathiaraj, H. Nyakotyo, Heliyon 3, e00285 (2017)
A.J. Leenheer, J.D. Perkins, M.F.A.M. van Hest, J.J. Berry, R.P. O’Hayre, D.S. Ginley, Phys. Rev. B 77, 115215 (2008)
Y.-T. Chuang, J.-W. Liou, W.-Y. Woon, Nanotechnology 28, 35603 (2017)
H. Kim, in Pulsed Laser Deposition of Thin Films, ed. by R. Eason (Wiley, Hoboken, 2006), pp. 239–260
J. Laube, D. Nbling, H. Beh, S. Gutsch, D. Hiller, M. Zacharias, Thin Solid Films 603, 377 (2016)
S. Sharma, S. Vyas, C. Periasamy, P. Chakrabarti, Superlattices Microstruct. 75, 378 (2014)
P. Banerjee, W.-J. Lee, K.-R. Bae, S.B. Lee, G.W. Rubloff, J. Appl. Phys. 108, 43504 (2010)
J.-H. Lee, K.-H. Ko, B.-O. Park, J. Cryst. Growth 247, 119 (2003)
S. O’Brien, M.G. Nolan, M. Çopuroglu, J.A. Hamilton, I. Povey, L. Pereira, R. Martins, E. Fortunato, M. Pemble, Thin Solid Films 518, 4515 (2010)
J. Pan, C. Zhao, X. Wei, C. Chi, W. Zhao, C. Song, Y. Zheng, C. Li, Nanotechnology 29, 085202 (2018). https://doi.org/10.1088/1361-6528/aaa327
K.H.L. Zhang, K. Xi, M.G. Blamire, R.G. Egdell, J. Phys. Condens. Matter 28, 383002 (2016)
H. Kawazoe, M. Yasukawa, H. Hyodo, M. Kurita, H. Yanagi, H. Hosono, Nature 389, 939 (1997)
J. Crêpellière, P. Lunca Popa, N. Bahlawane, R. Leturcq, F. Werner, S. Siebentritt, D. Lenoble, J. Mater. Chem. 31, 412–417 (2016)
H. Sun, M.A.P. Yazdi, F. Sanchette, A. Billard, J. Phys. D Appl. Phys. 49, 185105 (2016)
P. Lunca-Popa, J. Afonso, P. Grysan, J. Crêpellière, R. Leturcq, D. Lenoble, Sci. Rep. 8, 7216 (2018)
P. Lunca Popa, J. Crêpellière, R. Leturcq, D. Lenoble, Thin Solid Films 612, 194 (2016)
P. Lunca Popa, J. Crêpellière, P. Nukala, R. Leturcq, D. Lenoble, Appl. Mater. Today 9, 184 (2017)
T.-W. Chiu, K. Tonooka, N. Kikuchi, Thin Solid Films 516, 5941 (2008)
R.S. Ajimsha, K.A. Vanaja, M.K. Jayaraj, P. Misra, V.K. Dixit, L.M. Kukreja, Thin Solid Films 515, 7352 (2007)
H. Yanagi, K. Ueda, H. Ohta, M. Orita, M. Hirano, H. Hosono, Solid State Commun. 121, 15 (2001)
A.N. Banerjee, K.K. Chattopadhyay, Cent. Eur. J. Phys. 6, 57 (2008)
V. Rogé, N. Bahlawane, G. Lamblin, I. Fechete, F. Garin, A. Dinia, D. Lenoble, J. Mater. Chem. A 3, 11453 (2015)
Y. Zhang, G. Yousong, Y. Huang, X. Yan, Q. Liao, Controllable Synthesis, Structure and Property Modulation and Device Application of One-Dimensional Nanomaterials (World Scientific, Singapore, 2011)
T. Tharsika, A.S.M.A. Haseeb, S.A. Akbar, M.F. Mohd, Sabri, W.Y. Hoong, Sensors (Switzerland) 14, 14586 (2014)
J. Wang, Y. Lee, J.W.P. Hsu, J. Mater. Chem. C 4, 3607 (2016)
J. Husna, M.M. Aliyu, M.A. Islam, P. Chelvanathan, Energy Proc. 25, 55 (2012)
J.M. Shah, Y.L. Li, T. Gessmann, E.F. Schubert, J. Appl. Phys. 94, 2627 (2003)
A. Zekry, M. Abdel-Naby, H.F. Ragaie, F.E. El Akkad, IEEE Trans. Electron Devices 40, 259 (1993)
K. Kirah, A. Zekry, H.F. Ragaie, W.F. Fikry, J. Electrochem. Soc. 145, 332 (1998)
F.L. Schein, H. Von Wenckstern, M. Grundmann, Appl. Phys. Lett. 102, 092109 (2013)
Acknowledgements
This project was supported by the Luxembourg National Research Fund (FNR), Luxembourg (DEPTOS—Defect Engineering in p-type oxides semiconductors—project number C12/MS/3959502) and Luxembourg (Project PRIDE—project number 15/10935404/MASSENA). We would like to thank Mr. Patrick Grysan and Ms. Nathalie Valle for their valuable technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Afonso, J., Leturcq, R., Popa, P.L. et al. Transparent p-Cu0.66Cr1.33O2/n-ZnO heterojunction prepared in a five-step scalable process. J Mater Sci: Mater Electron 30, 1760–1766 (2019). https://doi.org/10.1007/s10854-018-0448-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0448-4