Abstract
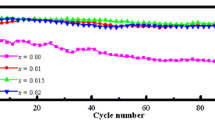
Rubidium modified Ni-rich LiNi0.8Co0.15Al0.05O2 cathode materials were successfully synthesized through a nano-milling assisted solid-state method. Rietveld refinement revealed that the Rb ions were incorporated into the lattice by replacing the original Li ions. It resulted in the enlarging of Li layer spacing together with the reducing of the thickness of the transition metal slab. Such changes in crystal structure led to the increase in the diffusion coefficient of Li ions in the lattice (DLi). Calculated diffusion coefficient presented the highest Li-ion diffusion coefficient of 1.54 × 10−10 cm2 s−1 for the sample Rb0.01Li0.99Ni0.8Co0.15Al0.05O2. It was also in good accordance with the changes in Li slab thickness. Improved electrochemical performance in specific capacity, capacity retention and rate performance were observed. Among the samples synthesized, the Rb0.01Li0.99Ni0.8Co0.15Al0.05O2 sample exhibited the highest initial discharge capacity and the best rate performance. It delivered specific discharge capacities of 190.5, 177, 169, 161 and 151 mAh g−1 at 0.1C, 0.2C, 0.5C, 1C and 5C, respectively. It also showed the best cycle stability with capacity retention of 91.31% after 100 cycles at 2C.








Similar content being viewed by others
References
C. Pan, K. Kou, G. Wu, Y. Zhang, Y. Wang, J. Mater. Sci. Mater. Electron. (2015). https://doi.org/10.1007/s10854-015-3752-2
Z. Jia, D. Lan, K. Lin, M. Qin, K. Kou, G. Wu, H. Wu, J. Mater. Sci. Mater. Electron. (2018). https://doi.org/10.1007/s10854-018-9909-z
L. Croguennec, M.R. Palacin, J. Am. Chem. Soc. (2015). https://doi.org/10.1021/ja507828x
A.A. Akl, S.A. Mahmoud, S.M. Al-Shomar, A.S. Hassanien, Mater. Sci. Semicond. Process. (2018). https://doi.org/10.1016/j.mssp.2017.10.007
H. Zhang, K. Karki, Y. Huang, M.S. Whittingham, E.A. Stach, G. Zhou, J. Phys. Chem. C (2017). https://doi.org/10.1021/acs.jpcc.6b10220
Z. Jia, K. Lin, G. Wu, H. Xing, H. Wu, Nano (2018). https://doi.org/10.1142/s1793292018300050
C. Pan, K. Kou, Y. Zhang, Z. Li, G. Wu, Composites. B (2018). https://doi.org/10.1016/j.compositesb.2018.07.019
J. Xu, F. Lin, M.M. Doeff, W. Tong, J. Mater. Chem. A (2017). https://doi.org/10.1039/c6ta07991a
A. Feng, G. Wu, Y. Wang, C. Pan, J. Nanosci. Nanotechnol. (2017). https://doi.org/10.1166/jnn.2017.13987
B.C. Park, H.B. Kim, H.J. Bang, J. Prakash, Y.K. Sun, Ind. Eng. Chem. Res. (2008). https://doi.org/10.1021/ie0715308
W. Liu, X. Tang, M. Qin, G. Li, J. Deng, X. Huang, Mater. Lett. (2016). https://doi.org/10.1016/j.matlet.2016.08.112
K. Du, H. Xie, G. Hu, Z. Peng, Y. Cao, F. Yu, ACS Appl. Mater. Interfaces (2016). https://doi.org/10.1021/acsami.6b05629
Y. Cho, J. Cho, J. Electrochem. Soc. (2010). https://doi.org/10.1149/1.3363852
Y. Xu, X. Li, Z. Wang, H. Guo, B. Huang, Mater. Lett. (2015). https://doi.org/10.1016/j.matlet.2014.12.093
Y.Q. Lai, M. Xu, Z.A. Zhang, C.H. Gao, P. Wang, Z.Y. Yu, J. Power Sources (2016). https://doi.org/10.1016/j.jpowsour.2016.01.079
Y. Huang, Y. Huang, X. Hu, Electrochim. Acta (2017). https://doi.org/10.1016/j.electacta.2017.02.067
G.R. Hu, X.R. Deng, Z.D. Peng, K. Du, Electrochim. Acta (2008). https://doi.org/10.1016/j.electacta.2007.10.040
B. Huang, X. Li, Z. Wang, H. Guo, Mater. Lett. (2014). https://doi.org/10.1016/j.matlet.2014.06.002
W. Liu, G. Hu, K. Du, Z. Peng, Y. Cao, Q. Liu, Mater. Lett. (2012). https://doi.org/10.1016/j.matlet.2012.05.100
W. Liu, G. Hu, K. Du, Z. Peng, Y. Cao, Surf. Coat. Technol. (2013). https://doi.org/10.1016/j.surfcoat.2012.11.057
B. Song, W. Li, S.M. Oh, A. Manthiram, ACS Appl. Mater. Interfaces (2017). https://doi.org/10.1021/acsami.7b00070
N. Wu, H. Wu, H. Liu, Y. Zhang, J. Alloys Compd. (2016). https://doi.org/10.1016/j.jallcom.2016.01.044
J. Duan, C. Wu, Y. Cao, K. Du, Z. Peng, G. Hu, Electrochim. Acta (2016). https://doi.org/10.1016/j.electacta.2016.10.158
S. Muto, K. Tatsumi, Y. Kojima, H. Oka, H. Kondo, K. Horibuchi, Y. Ukyo, J. Power Sources (2012). https://doi.org/10.1016/j.jpowsour.2012.01.071
A.H. Tavakoli, H. Kondo, Y. Ukyo, A. Navrotsky, J. Electrochem. Soc. (2013). https://doi.org/10.1149/2.059302jes
P. Yue, Z. Wang, J. Wang, H. Guo, X. Xiong, X. Li, Powder Technol. (2013). https://doi.org/10.1016/j.powtec.2012.12.061
X. Li, Z. Xie, W. Liu, W. Ge, H. Wang, M. Qu, Electrochim. Acta (2015). https://doi.org/10.1016/j.electacta.2015.06.099
H. Xie, K. Du, G. Hu, Z. Peng, Y. Cao, J. Phys. Chem. C (2016). https://doi.org/10.1021/acs.jpcc.5b12407
K. Saravanan, M.V. Reddy, P. Balaya, H. Gong, B.V.R. Chowdari, J.J. Vittal, J. Mater. Chem. (2009). https://doi.org/10.1039/b817242k
G. Wu, H. Wu, K. Wang, C. Zheng, Y. Wang, A. Feng, RSC Adv. (2016). https://doi.org/10.1039/c6ra11771f
S. Lim, C.S. Yoon, J. Cho, Chem. Mater. (2008). https://doi.org/10.1021/cm8006364
G. Arnold, J. Garche, R. Hemmer, S. Ströbele, C. Vogler, M. Wohlfahrt-Mehrens, J. Power Sources (2003). https://doi.org/10.1016/s0378-7753(03)00241-6
Y. Ding, Y. Jiang, F. Xu, J. Yin, H. Ren, Q. Zhuo, Z. Long, P. Zhang, Electrochem. Commun. (2010). https://doi.org/10.1016/j.elecom.2009.10.023
R. Dominko, M. Bele, J.M. Goupil, M. Gaberscek, D. Hanzel, I. Arcon, J. Jamnik, Chem. Mater. (2007). https://doi.org/10.1021/cm062843g
Y. Zhou, J. Wang, Y. Hu, R. O’Hayre, Z. Shao, Chem. Commun. (Camb) (2010). https://doi.org/10.1039/c0cc01721c
A.S. Hassanien, A.A. Akl, A.H. Sáaedi, CrystEngCommun (2018). https://doi.org/10.1039/c7ce02173a
N. Recham, L. Dupont, M. Courty, K. Djellab, D. Larcher, M. Armand, J.M. Tarascon, Chem. Mater. (2009). https://doi.org/10.1021/cm803259x
A.A. Akl, A.S. Hassanien, Superlattices Microstruct. (2015). https://doi.org/10.1016/j.spmi.2015.05.011
K. Kang, Y.S. Meng, J. Bréger, C.P. Grey, G. Ceder, Science (2006). https://doi.org/10.1126/science.1122152
Z. Zhang, D. Chen, C. Chang, RSC Adv. (2017). https://doi.org/10.1039/c7ra10053a
L. Guan, P. Xiao, T. Lv, D. Zhang, C. Chang, J. Electrochem. Soc. (2017). https://doi.org/10.1149/2.1731713jes
N. Li, Y.S. He, X. Wang, W. Zhang, Z.F. Ma, D. Zhang, Electrochim. Acta (2017). https://doi.org/10.1016/j.electacta.2017.01.137
B.H. Toby, J. Appl. Crystallogr. (2001). https://doi.org/10.1107/S0021889801002242
W.S. Yoon, K.Y. Chung, J. McBreen, X.Q. Yang, Electrochem. Commun. (2006). https://doi.org/10.1016/j.elecom.2006.06.005
X. Li, W. Ge, H. Wang, X. Yan, B. Deng, T. Chen, M. Qua, Electrochim. Acta (2017). https://doi.org/10.1016/j.electacta.2016.12.138
M. Guilmard, C. Pouillerie, L. Croguennec, C. Delmas, Solid State Ion (2003). https://doi.org/10.1016/S0167-2738(03)00106-1
M. Guilmard, A. Rougier, M. Grüne, L. Croguennec, J. Power Sources (2003). https://doi.org/10.1016/s0378-7753(03)00012-0
Y. Gao, X. Wang, J. Ma, Z. Wang, L. Chen, Chem. Mater. (2015). https://doi.org/10.1021/acs.chemmater.5b00875
Z.Y. Li, J. Zhang, R. Gao, H. Zhang, Z. Hu, X. Liu, ACS Appl. Mater. Interfaces (2016). https://doi.org/10.1021/acsami.6b04073
Z. Huang, Z. Wang, X. Zheng, H. Guo, X. Li, Q. Jing, Z. Yang, Electrochim. Acta (2015). https://doi.org/10.1016/j.electacta.2015.09.151
D. Mohanty, J. Li, D.P. Abraham, A. Huq, E.A. Payzant, D.L. Wood, C. Daniel, Chem. Mater. (2014). https://doi.org/10.1021/cm5031415
P. Gao, R. Ishikawa, E. Tochigi, A. Kumamoto, N. Shibata, Y. Ikuhara, Chem. Mater. (2017). https://doi.org/10.1021/acs.chemmater.6b03659
J.R. Croy, K.G. Gallagher, M. Balasubramanian, B.R. Long, M.M. Thackeray, J. Electrochem. Soc. (2013). https://doi.org/10.1149/2.049403jes
M. Sathiya, A.M. Abakumov, D. Foix, G. Rousse, K. Ramesha, M. Saubanere, M.L. Doublet, H. Vezin, C.P. Laisa, A.S. Prakash, D. Gonbeau, G. VanTendeloo, J.M. Tarascon, Nat. Mater. (2015). https://doi.org/10.1038/nmat4137
L. Pan, Y. Xia, B. Qiu, H. Zhao, H. Guo, K. Jia, Q. Gu, Z. Liu, J. Power Sources (2016). https://doi.org/10.1016/j.jpowsour.2016.07.064
Y. Zhao, L. Peng, B. Liu, G. Yu, Nano Lett. (2014). https://doi.org/10.1021/nl5008568
Funding
The research was supported by Science and Technology Commission of Shanghai Municipality (14520503100 and 201310-JD-B2-009) and Shanghai Municipal Education Commission (15ZZ095).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dong, J., Xiao, P., Zhang, D. et al. Enhanced rate performance and cycle stability of LiNi0.8Co0.15Al0.05O2 via Rb doping. J Mater Sci: Mater Electron 29, 21119–21129 (2018). https://doi.org/10.1007/s10854-018-0260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0260-1