Abstract
Narrow width of optical absorption of conducting polymers and photons energy losses have been the challenges for fabricating highly efficient thin-film organic solar cell. Nickel-doped silver nanoclusters (Ni/Ag NCs) are employed here to capture more photons using polymers blend solar absorber medium to improve solar cell performances. The poly-3-hexylthiophene and (6-6)phenyl-C61-butyric acid methyl ester molecules blend were used a solar absorber layer in this investigation. The solar cells fabricated with NCs exhibited enhanced opt-electronic properties compared to the reference solar cell. Consequently, the experimental results suggest that the power conversion efficiency (PCE) has substantially increased with the incorporations of NCs in absorber layer, which is dependent on the concentrations of NCs in the medium. The maximum PCE achieved, in this work, is \(\eta \) = 6.2% at 2% of NCs by weight, which has exhibited to the lowest energy losses compared to other doping levels. This improvement in PCE is attributed to the occurrence of local surface plasmon resonance effect due to the inclusion of Ni/Ag NCs in polymer matrix. The results provide valuable insights on the use of Ni/Ag NCs for efficient photons capture in thin-film polymers blend medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solution-processed thin-film organic solar cells (TFOSCs) have garnered significant attention in the research community due to their mechanical flexibility, lightweight, and inexpensive fabrication methods [1,2,3,4]. However, there are still challenges that need to be addressed in the area of improving optical absorption band width, charge transport processes, and life time of TFOSC without compromising the low-cost device fabrication. In this study, a number of approaches have been proposed to optimize photon energy harvesting, inter-facial engineering, and controlling film morphology in TFOSCs through the incorporation of various materials, such as metal nanoparticles (NPs), graphene, carbon nano-tubes, and solvent additives [5, 6]. One key component, in achieving high-power conversion efficiency, in TFOSCs structure is the nature of solar absorber for effective photons capture and the generation of free charge carriers [9,10,11]. Increasing the thickness of the thin-film solar absorber layer is one of the direct mechanisms to enhance optical absorption and harvest more photons. However, this approach promotes high charge recombination due to short diffusion length of exciton in the polymer medium (\(\simeq \) 10 nm) [12,13,14,15]. Consequently, solar cells with relatively high active layer thickness’s suffer from significant loss of photo-generated current through radiative and non-radiative charge recombinations before reaching to the donor/acceptor interface. Therefore, to address this challenge, researchers have explored on the use of metal NPs at different functional layers of TFOSCs. Metal NPs enhance light absorption via light scattering processes that increase optical path length leading to trapping in the photoactive medium. This resulted in improved performance indicators across all solar cell parameters [13, 16]. There are several research reports on the positive impact of metal NPs such as silver, copper, and gold incorporated into photoactive layer of a solar cells, which are characterized by enhanced electrical conductivity of the thin-film organic semiconductor medium [7, 8, 17,18,19,20]. Additionally, the occurrence of surface plasmonic resonance (SPR) of the metal NPs can significantly improve the optical absorption of the semiconductor medium [21] by promoting light scattering and electron band-to-band transition. A thorough understanding of the physics behind the local surface plasmonic resonance (LSPR) phenomenon is crucial for the development and implementation of plasmonic NPs for various applications. To achieve the desired adaptation between film absorption and SPR property of the metal nanoparticles, certain physical features such as size, morphology, location, and composition of noble metal NPs must be precisely designed, taking into account the permittivity of the surrounding medium [22]. An excellent device performance is reported from use of poly-(3-hexylthiophene) (P3HT) and non-fullerene acceptor [23], which is an important development on the application of stable donor polymer P3HT. On the other hand, metal nanoparticles such as gold (Au) are reported to make notable influence on the optical absorption and subsequent improvement in power conversion efficiencies (PCE) of solar cells, upon the integration of plasmonic gold (Au) nanoparticles into OPV structures, consisting of P3HT and [6,6]-phenyl C61 butyric acid methyl ester (PCBM) [24,25,26,27]. However, the utilization of materials such as gold for large-scale device production remains a challenge due to their limited supply and high cost. In this study, Ni/Ag NCs have been used for the first time in the P3HT and PC61BM blend solar absorber known as P3HT:PCBM matrix or any other conjugated polymer blend to the knowledge of the authors. The inclusion of the NCs in a polymer blend has positively impacted the optical absorption and the device performance much better than reported in the literature for the same polymer blends. Therefore, the results of the investigation indicated that significant improvement in PCE is recorded due to the presence of Ni/Ag NCs in device functional layers. Details of the results are discussed in the following sections.
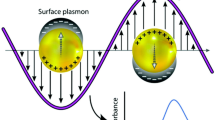
a A schematic diagram illustrating the process of electron–hole pair generation and inter-band transition at the local surface plasmon resonance in pure metal and hybrid metal nanocomposites. The diagram shows how the incident light induces oscillations of electron clouds (left), leading to a decline in LSPR and the generation of a population of hot carriers with various energies (right). b How inter-band transitions are a result of higher energy photons (left) and directly produce electron–hole pairs (right) [28].
Fundamentals of surface plasmonic
The origin of SPR is the coherent oscillation of free electrons on the surface of metal NPs by the actions of the incident electromagnetic field. This oscillation arises from the induced dipole moments as the result of surface electrons displacement relative to the nuclei due to changing electric field of the incident electromagnetic wave (see Fig. 1a). The restoring force of the induced moment causes the electron cloud to oscillate against the oppositely charged nuclei at a specific frequency [29, 30]. A resonance occurs when the frequency of oscillation coincides with the incident photons. On the other hand, such interactions between electron plasma and incident radiation could cause excitation of metals through electron band-to-band transition (see Fig. 1b) and contribute to optical absorption. The intense electromagnetic field formed near the metal NPs, as the result of the interaction, would cause emission of light at a frequency similar to the incident photons and is scattered in all directions [31]. Therefore, the collective oscillation of electrons on the surface of NPs produce a high extinction cross-section of metal NPs in the form of scattering and absorption. Furthermore, the oscillating electrons lose some of their energy in the form of heat due to their continuous mechanical oscillations. Nonetheless, the active area of the metal nanoparticle that scatters light is defined by the scattering cross-section \(\sigma _{sc}\) [32] while the absorption cross-section is denoted by \(\sigma \)(abs). Hence, the total extinction cross-section coefficient of the metal NPs in the surrounding medium can be expressed by the Espinosa Eq. [33].
The absorption and scattering cross-sections are derived from the classical electromagnetic theory using spherical metal NPs with diameters below the light penetration depth. By employing quasi-static approximation method, the absorption cross-section (\(\sigma _{abs}\)) and scattering cross-section (\(\sigma _{sca}\)) are given by:
where wave vector k is defined as k = 2\(\pi \)/\(\lambda \), \(\lambda \) is the wavelength of the incident wave. In this context, \(\varepsilon \)(\(\omega \)) represents the dielectric constant or permittivity of the metal nanoparticle, while a refers to the radius of the nanoparticle. The dielectric constant \(\varepsilon _d\) stands for the medium that surrounds the nanoparticle, as cited in reference [34]. In the realm of physics, the permittivity \(\varepsilon \)(\(\omega \)) can be expressed as a composite of two distinct components, namely the real and imaginary parts. The real part, often referred to as the resonance peak, serves as a marker of the position of the permittivity. On the other hand, the imaginary part conveys information about the de-phasing. It is important to note that these two components work in tandem to fully characterize the permittivity.
The permittivity of metal nanoparticles in Eq. (4) is further rearranged and simplified into:
where \(\omega _p\) and \(\gamma _b\) are the plasmonic frequency and the damping constant of metal nanoparticles, respectively. These parameters are defined by the following equations:
Thus, n is the density of electrons, e is electron charge, m is mass of the electron, and \(\varepsilon _0\) is the permittivity of the free space. Therefore, the symbol \(\gamma _b\) refers to the damping constant, which is represented by the fermi velocity and mean free path of the electrons \(l_m\) [35].
Materials and methods
Materials
The chemicals used in the synthesize of Ni/Ag NCs were obtained from commercial sources and used as delivered. These are silver nitrate (AgNO\(_3\)), nickel nitrate (Ni(NO\(_{3}\))\(_2\)) (\(\ge \)99.0%), and sodium hydroxide (>99.98% NaBH4) are purchased from Merck. The photoactive layer in the BHJ absorber consisted of polymers such as poly(3-hexylthiophene) (P3HT) as the electron donor and (6-6)phenyl-C61-butyric acid methyl ester (PC61BM) as the electron acceptor which were obtained from Ossila Ltd. (UK). The PEDOT:PSS and chloroform solvent were procured from Sigma-Aldrich.
Synthesis and characterizations of nickel-doped silver NCs (Ni/Ag)
In this study, nickel-doped silver (Ni/Ag) was synthesized using a chemical reduction method that has been previously reported in the literature [36]. Initially, solutions of nickel nitrate (0.44 g), silver nitrate (0.13 g), and sodium borohydride (NaBH4) (0.57 g) were prepared by dissolving each compound in 50 mL of deionized water in separate flasks. The solutions were stirred for 10 min before the silver nitrate solution was added dropwise to the nickel nitrate solution under magnetic stirring. The resulting mixture was further stirred on a hot plate with a magnetic stirrer for 20 min, followed by the addition of NaBH4 solution. The mixture was then stirred continuously for 3 h at 40 \(^\circ \)C. The resulting solution was purified several times using deionized water to eliminate the sodium nitrate agent, and the precipitate was then dehydrated for 2 h at 70\(^\circ \)C under vacuum. This resulted in the synthesis of nickel-doped silver powder. To study morphology and photosensitive properties of the synthesized Ni/Ag NCs, we used ultraviolet–visible (UV–Vis) absorption spectra, high-resolution tunnelling and scanning electron microscopy (HRTEM and HRSEM), energy-dispersive X-ray (EDX), and X-ray diffraction (XRD) measurements.
Device preparations
Thin-film organic solar cells were fabricated on transparent indium tin oxide (ITO)-coated glass substrates. The absorber layers are composed of polymers blend of P3HT:PC61BM with/without Ni/Ag NCs. Initially, the substrates were partially etched with a warm acid solution consisting of hydrochloric acid, water, and nitric acid (HCL:H\(_2\)O:HNO\(_3\)) at a concentration of 48%:48%:4%) by volume. The substrates were then cleaned in an ultrasonic bath with deionized water, acetone, and isopropanol for 15 min, followed by drying in an oven at 100\(^\circ \)C for 20 min. Next, a thin hole transport layer PEDOT:PSS was spin-coated onto the substrates, followed by annealing in an oven under an ambient atmosphere at 100\(^\circ \)C for 20 min. The solar absorber medium comprising of P3HT and PC61BM polymers was prepared by dissolving them in chloroform solvent at a concentration of 20 mg/ml and using a 1:1 stoichiometric ratio by weight. The metal NCs are dispersed in the solution at the concentration 1%, 2%, 3%, and 5% by weight relative to the weight of the polymers blend. The solutions were stirred on a hot plate at 40\(^\circ \)C for 4 h to ensure proper miscibility of the polymers. The resulting blends were spin-coated on top of the dried PEDOT:PSS layer at 1200 rpm for 40 s, followed by dehydration under a nitrogen atmosphere for 10 min at 90\(^\circ \)C. The samples were then transferred onto the vacuum deposition chamber for the thermal evaporation process for the electron transfer layer lithium fluoride LiF and the electrode aluminium (Al). Thin layers of LiF (0.5 nm) and Al (100 nm) were deposited on top of the samples. The resulting devices had the following structure: ITO/PEDOT: PSS/P3HT:PCBM:Ni/Ag NCs/LiF/Al. The devices were further annealed for 5 min. The performance of the devices was determined by measuring the electrical properties using a Keithley HP2420 source meter integrated with a solar simulator (model SS50AAA). The simulator is operating at AM1.5 and 100 mW cm\(^{-2}\) of light intensity. A schematic diagram for the conventional device structure with the combination of Ni/Ag NCs is displayed in Fig. 2 (below).
Results and discussion
Characterizations of Ni/Ag NCs
The optical properties of Ni/Ag NCs
The optical properties of the Ni/Ag NCs were examined through the use of UV–Vis spectrometer. The measured UV–Vis spectra of the newly synthesised metal NPs taken in a deionized water suspension are given in Fig. 3(a and b). As illustrated in Fig. 3(a), the absorbance of nickel-doped NC exhibited absorbency within the range of 350–900 nm, with a peak located at 460 nm, and a tail extending into the near-infrared region of the electromagnetic spectrum. This significant broadening of the absorption spectra of the synthesized nano-composites indicates the presence of multiple sizes and shapes of metal nanoparticles, which is expected to enhance the optical extinction coefficient of the solar absorber through photons absorption in small volumes (LSPR modes) and light scattering in larger sizes to the surrounding medium [37].
Tauc’s model is also employed to determine the energy band gap, Fig. 3b. Tauc’s model relies on the absorption coefficient of the materials \(\alpha \), which is related to the optical energy band gap, E\(_g\). The relationship between \(\alpha \) and E\(_g\) is described by equation shown below:
Here \(\beta \) is the photon’s energy and is defined by \(\beta \) = hc/\(\lambda \), where h stands for Plank’s constant, c is the speed of light, and \(\lambda \) is the wavelength of the photon. The constant parameter \(\gamma \) characterizes the optical frequency range, and the index n is used to describe the electronic transition conditions, taking on a value of 1/2 for direct transitions or 2 for indirect transitions. The absorption coefficient \(\alpha \) is a crucial parameter in this model. By analysing the plot (\(\alpha \)h\(\upsilon \)) vs (h\(\upsilon \)) and extrapolating a line tangent to the curve (\(\alpha \)h\(\upsilon \))\(^2\)=0, it was possible to determine the optical energy gap to be 1.67 eV. This value of optical band gap is anticipated to have a notable impact on the energy harvesting capabilities of the photoactive layer of solar cells, particularly for longer wavelength electromagnetic photons [38, 39].
Morphology of the Ag/Ni NCs
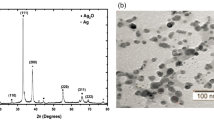
The morphology of the Ni/Ag NCs in powder form was investigated using high-resolution transmission and scanning electron microscopies (HRTEM and HRSEM), respectively. These spectrometers provide information such as shape, size, crystallinity, and elemental mapping of nanocomposite. The HRTEM images taken from nickel-doped silver NCs powders are presented in Fig. 4, where Fig. 4a and c illustrates the formations of various sizes and shapes of NCs. It has been noted that there is nano-sphere-like structure as evidenced in Fig. 5a, which exhibited various sizes in the medium. The size variations have a significant influence on energy harvesting process through the occurrence of SPR and LSPR effects. The HRSEM elemental mapping provided in Fig. 4d demonstrates an even distribution of nickel and silver elements within the medium, indicating the good participation of the elements in the formation of Ni/Ag NCs. Furthermore, the energy-dispersive X-ray (EDX) image in Fig. 4d reveals the nickel and silver concentrations in the metal NCs corresponding to 64.98% and 35.02%, respectively, at a 2:1 ratio. This finding confirms the purity of the synthesized metal NCs and their formation at the required ratio throughout the medium, which is beneficial for influencing molecular crystallinity and energy harvesting in solar cell devices. The crystalline fringes observed in HRTEM imaging are depicted in Fig. 4b, which revealed an atomic lattice spacing of 0.226 nm, consistent with a cubic crystal structure of the metal NCs found using XRD measurements. According to XRD data, the d-spacing of the Ni/Ag NCs was calculated using the Bragg equation and found to be d = 0.229 nm, which is in good agreement with the values derived from HRTEM images of 0.226 nm, which corresponds to the (111) atomic plane of the Ni/Ag NCs [40].
a High-resolution transmission electron microscopy image of Ni/Ag powder representing different shapes and sizes of the NCs, b the crystallite fringes taken from Ni/Ag powder indicating lattice spacing and crystallite of Ni/Ag NCs, c scanning electron microscopy (SEM) image showing the morphology of Ni/Ag NCs in powder form, and d SEM image background and the energy-dispersive X-ray analysis taken from Ni/Ag powder indicating the elemental composition.
XRD study of Ni/Ag
To examine the crystallographic structure and morphological characteristics of Ni/Ag NCs, X-ray diffraction (XRD) analysis was carried out. The measured XRD spectrum, depicted in Fig. 5, was obtained by scanning the sample from angles 20\(^\circ \) to 90\(^\circ \) at 2 theta degrees. The diffraction peaks observed at 2\(\Theta \) angles indicated the presence of five prominent peaks at values of 38.29\(^\circ \), 44.42\(^\circ \), 64.56\(^\circ \), 77.56\(^\circ \), and 81.60\(^\circ \). These diffraction peaks corresponded to the reflections of the X-ray beam from planes (111), (200), (220), (311), and (222), respectively. Our findings indicated that Ni/Ag NCs exhibited a cubic crystal structure with cell parameters a = 4.0772 A\(^{0}\), b = 4.0772 A\(^{0}\), and c =4.0772 A\(^{0}\) with alpha = 90\(^\circ \), beta = 90\(^\circ \), and gamma = 90\(^\circ \)[41]. Using Debye–Scherer’s following expression, the crystalline size D of the Ni/Ag NCs was calculated.
The Scherer constant (K) was determined to be 0.89, and the wavelength of the X-ray \(\lambda \) was 1.54 Angstroms. \(\beta \) and \(\theta \), the full width at half maximum (FWHM) of the diffraction peak measured in radian and their corresponding maximum peak angles determined by using Bragg’s equation in degree, were also taken into consideration. The calculated average size for the powder crystals was 50.8 nm, indicating the significant light scattering effect of plasmonic Ni/Ag that influenced the energy harvesting of the photoactive layer. Table 1 summarizes the results obtained from the X-ray diffraction analysis of Ni/Ag NCs.
In additional, the crystallographic structure of Ni/Ag NCs was evaluated by dislocation density (\(\delta \)), micro-strain (\(\varepsilon \)) and stress (\(\sigma \)) were calculated by employing Hooke’s law on the measured XRD data, can be determined by the following equations:
However, the micro-strain (\(\varepsilon \)) and stress (\(\sigma \)) of Ni/Ag NCs are linearly increase with decrease crystal sizes (see the table). Furthermore, the crystallite sizes of Ni/Ag NCs are found to be between 26 nm and 70 nm, which are designed in smaller structure. This is suitable for photoactive layer polymer blend for improved charge transport processes. The stress can be determined from the ascribed micro-strain, and the value of C = 1.46 ± 10\(^{10}\) Nm\(^{-2}\) taken from the bulk Young’s modulus [42, 43].
Device characterizations
Optical properties of thin-film solar absorbers
The optical properties of solar absorber layers are an important mechanism to investigate the effect of plasmonic Ni/Ag NCs on the properties of the polymers films. The UV–Vis measurements were conducted both on Ni/Ag NCs doped thin-film absorber and reference film without Ni/Ag NCs. The measured UV–Vis spectra taken from the absorbers films with/without Ni/Ag NCs are presented in Fig. 6a. The spectra are dominated by the absorption of PT3HT:PCBM blend centred around 515-nm peak accompanied by vibronic shoulders. However, NCs doped films generally showed strong intensities compared to the reference film and dependent on the concentration of metal NCs in the medium. According to the absorbancy of the Ag/Ni NC provided in Fig. 3a, exhibited absorbency within the range of 350–650 nm, with a peak centred at 460 nm, and tail that extended to the near-infrared region of the electromagnetic spectrum. The broadened absorption of the synthesized NCs implies the existence of multiple sizes and shapes of metal nanoparticles, which is expected to increase the optical extinction coefficient of the solar absorber via photon absorption for small volumes (LSPR modes) and light scattering to the surrounding medium for large sizes. As expected, the incorporation of Ni/Ag NCs into P3HT:PCBM blend solar absorber was significantly enhanced the absorbency and the intensity of the films as presented in Fig. 6a. The spectra from the films clearly showed the signature of Ni/Ag NCs in terms of location and the intensity absorption peaks. The absorption spectra of Ni/Ag NCs doped films exhibited changes in peak intensity and FWHM broadening, a blue shift, damping, due to the occurrence of surface plasmon resonance. The extent of the changes is dependent on the proportion of nickel in the medium, which is in good agreement with classical predictions, by assuming a Ni-core/Ag-shell geometry. These observations are similar to the findings of Gaudery and co-authors, in which they investigated the optical properties of mixed clusters focusing on the behaviour of surface plasmon resonance of Ni/Ag NCs in a spectral range comparable to that of pure silver clusters. Notably, the surface plasmon resonance in Ni/Ag NCs was observed to be considerably broadened by increasing the silver concentration similar to our observations in the absorption spectra of doped samples. Their study also found that the absorption spectra of Ni/Ag NCs tend to be more blue shifted as the concentration of nickel increases providing a relevant comparison to our study [44]. The new absorption peaks near 400 nm for Ni/Ag NCs solar cells are associated with band-to-band electron transition of silver molecules [45]. Moreover, the presence of the vibronic shoulder of P3HT around 600 nm of wavelength for all doped concentrations of Ni/Ag NCs solar cells suggests that the amount of NCs used in this experiment did not alter the crystal structure of the donor polymer. It is to be noted that the near-field enhancement on the site of NCs is the most dominant effect for Ni/Ag NCs on the fabricated solar cells. This is attributed to the occurrence of LSPR, enhanced exciton dissociations and light trapping via scattering processes, which are collectively improve device performances.
Solar cell performances
The performance of solar cells in converting incident photons into electricity was investigated by analysing the measured current density–voltage (J–V) characteristic curves. The J–V curves were taken from solar cells fabricated under different concentrations of NCs in the solar absorber layer. According to the results presented in Fig. 7, which is evident that there is a surge of photocurrent under the influence of NCs in the device structure. The short current density (J\(_{sc}\)) significantly high at 1% and 2% of Ni/Ag NCs, but the J\(_{sc}\) was slightly decreased at 3%, and then further dropped when the concentration is increased to 5% by weight. This results suggest that the high dopant ratios of metal NCs in the medium have a negative impact on solar cell performance. This is evident by the reduction in PCE from 6.2% to 2.8%, which is accompanied by high series resistance (R\(_s\)). The maximum J\(_{sc}\) recorded in this experiment was 17.8 (mAm\(^{-2}\)) for the 2% Ni/Ag by weight, followed by 15.2 (mAm\(^{-2}\)) for both 1% and 3% concentrations. However, the lowest J\(_{sc}\) measured in this study was 10.7 (mAm\(^{-2}\)) for 5% concentration, which was higher than 10.2 (mAm\(^{-2}\)) for the reference solar cell. On the other hand, the fill factor (FF) of the solar cells with the incorporation of Ni/Ag was also increased from 51.2% for 0% to 58.5% and 58.3% for 1% and 2% concentration, respectively. However, the FF at 3% and 5% NC concentration was dramatically dropped into 51.0% and 44.2%, respectively. The reduced FF, at 3% and 5% concentrations, would suggest that there must have been leakage currents and charge recombination enhancement in the devices. Thus, the high concentration of the metal NCs in the polymer medium might have caused several defect clusters that promote charge recombinations and prevent the free charge collections. The summary of the J–V characteristic data is presented in Table 2. The optimum concentration of Ni/Ag NCs in this investigation was found to be 2%, which resulted in the best performance with a PCE = 6.2%. The open-circuit voltage (Voc) measured for the solar cells was observed to increase from 0.62 V for pristine to 0.63 V for 1% and then suddenly dropped to 59 V for 2% Ni/Ag NCs concentration. This is due to the interaction of the polymers with the nanoparticles, which resulted in changes in the energy levels that affected the energy band gap. However, as the concentration increases, more Ag/Ni-related defect cluster could have formed that hindered the charge dissociations and transportations. Consequently, this has reduced the measured photocurrent beyond 2% doping level in polymer medium. Moreover, higher concentrations of NCs in the absorber layer would result more metal particles at the interface between active layer and electrodes and causing leakage current [46, 47] and reduce the device FF. This is consistent with measured data provided in Table 2. According to Ohm’s law, shunt current increases as shunt resistance decreases, leading to a significant leakage and reduced V\(_{oc}\) and FF of the solar cell [48]. Equation12 defines:
where V is the applied voltage, J is the current density, J\(_{sh}\) is the shunt current, and R\(_s\) and R\(_{sh}\) are the series and shunt resistances, respectively. Moreover, as shown in result, Table 2, the collected series resistances from J–V measurements for 0%, 1%, 2%, 3%, and 5% of Ni/Ag dopant concentrations range from 1.86 \(\Omega \)cm\(^2\) to 13.69 \(\Omega \)cm\(^2\), respectively. On the other hand, the values of R\(_{sh}\) vary from 105.1 \(\Omega \)cm\(^2\) to 188.6 \(\Omega \)cm\(^2\), in the chosen range of NC concentration. This outcome suggests that an increase in Ni/Ag NCs concentration is unfavourable for the solar cell performances.
The effect of Ni/Ag NCs on charge recombination mechanisms was also investigated using the same calculations based on Tauc’s model as mentioned in the previous section. The study was based on the general energy losses definition of the solar cells in the form of E\(_{loss}\)= E\(_g\)- qV\(_{oc}\), where E\(_g\) is the optical energy gap of the active thin films, and q is the elementary charge. The measured energy gap values for the solar cells with and without Ni/Ag NCs are presented in Fig. 6b. The incorporation of synthesized Ni/Ag NCs was found to be significantly reduce the optical band gap of the fabricated thin films from 1.59 eV to 1.40 eV (see Table 2). The energy losses calculated based on these values were found to be ranging from 0.98 eV for the reference to 0.81 eV 2% doping concentrations, respectively. As per the existing literature, solar cells exhibiting low-energy losses tend to perform better, resulting in improved J\(_{sc}\), FF, and higher PCE due to a reduction in charge recombination.
Charge transport properties of the conventional solar cells
The charge transport properties in Ag/Ni-doped polymer solar absorber were investigated based on the measured space charge limited current (SCLC) under dark conditions. The dark current is an ideal environment by reducing the impact of illumination on the charge transport processes and understand the true nature the polymer medium. Figure 8a shows lnJ versus V curves taken from newly fabricated solar cell devices, which clearly shows high current in dark conditions for NC-doped solar cells. Figure 8b displays the SCLC data collected from the forward bias side of the dark current, which started from the injection limited region (1.4 V) of the device and ended at the point where the current reaches saturated. Then, the mobilities of the solar cells were determined via the current density of the devices using Mott–Gurney’s law, as provided below (13):
where \(\varepsilon _0\) is the permittivity of the free space = 8.85 \(\times \) 10\(^-{12}\) F/m, \(\varepsilon \) is the relative permittivity of the medium, \(\mu \) is the mobility, L is the active layer thickness = 100 nm, V is the voltage drop across the sample which is corrected from the deference between the applied V\(_{apl}\), and build-in voltage V\(_{bi}\) [49, 50].
It is also important to mention that, the mobility \(\mu \) of the solar cell in Poole–Frenkel’s equation [51] is strongly correlated with the electric field across the device as follows:
where \( \gamma \) is the field activation factor, and \( \mu _0 \) is the zero-field mobility. The trap free field-dependent SCLC was then calculated by combining Eqs. (15) and (13), as given in Eq. (16).
The model of Mott–Gurney’s assumes constant charge mobility in the medium, free of traps, with an ideal ohmic contact at the cathode and anode. For the pristine solar cell, the resultant zero-field mobility measured was 1.17\(\times \)10\(^{-3}\) (cm\(^2\) S\(^{-1}\) V\(^{-1}\)) (see Table 3), which is effectively enhanced to 1.67\(\times \)10\(^{-3}\) (cm\(^2\) S\(^{-1}\) V\(^{-1}\)) and 1.73\(\times \)10\(^{-3}\) (cm\(^2\) S\(^{-1}\) V\(^{-1}\)) for 1% and 2% NC doping levels, respectively. However, as the concentration of Ni/Ag NC increases to 3%, then the zero-field mobility decreased to 9.32\(\times \)10\(^{-4}\) (cm\(^2\) S\(^{-1}\) V\(^{-1}\)), and further dropped to 4.13\(\times \)10\(^{-4}\) (cm\(^2\) S\(^{-1}\) V\(^{-1}\)) at 5% NC by weight. It has to be noted here that the higher zero-field mobility leads to better efficiency of the solar cell while lower mobility reduces charge collections as evidenced by the drop in PCE to 2.8% at 5% doping level. Furthermore, the zero-field mobility decreases as the concentration of the metal NCs increases; this suggests that more defect cluster could be formed that prevents the charge transport as the concentration of NC increases beyond the optimum limit.
In the case of the reference P3HT:PECBM photoactive layer, the effective charge transport process originated from the polymer film morphology. Better polymer morphology would lead to enhanced collection of photocurrent becuase of good molecular miscibility and large acceptor/donor interfacial area throughout the medium [52]. Thermal annealing is one of the mechanisms to improves polymers crystallization and promotes charge transfer between molecules. The discrepancy in mobility between electrons and holes can lead to an imbalance charge transport, which can increase for non-geminate charge recombination in polymer solar cells [53, 54]. The use of plasmonic metal NPs, in a polymer photoactive layer, increases not only the optical absorption the polymer film but also has a great impact on the charge mobility in the medium. Omrani et al. have reported that plasmonic metal NPs can introduce hopping sites for holes by creating dopant states in the polymer band gap space and increasing mobility [55, 56]. The solar cell’s field activation factor (\(\gamma \)) provided in Table 3, is a mathematical parameter utilized to approximate the ratio of charge recombination to that charge generation before collection by the electrodes. A lower value of the \(\gamma \) implies greater mobility of charge carriers, which consequently lowers the probability of non-geminate energy losses inside the polymer medium [56].
Table 3 summerizes the values of zero-field mobility and field activation factors derived from space charge limited current. Best value of charge mobiity recorded in this investgation was 1.73\(\times \)10\(^{-3}\) (cm\(^2\) S\(^{-1}\) V\(^{-1}\)) at 2% NC concetration in the medium, which corresponds to best PCE. This clear indicates the substantial influence of Ni/Ag NCs on the charge transport process, which ultimately boosted the PCE of the solar cells. On the other hand, the left flank of Fig. 8a representing the reverse saturation current, can be originated from electrode charge injection or/and thermal generation from the polymer medium, under dark condition. The current in reverse bias increases till the optimum concentration of Ag/Ni in solar absorber and then drops at higher doping levels, suggesting that the amount of current even in reverse bias is dependent on improved charge transport processes in the medium. More charges are collected at the optimum doping level, which is consistent with the charge transport parameters derived from space charge limited current measured in the forward bias and dark condition.
Conclusion
In conclusion, the incorporation of Ni/Ag NCs in the absorber layer of bulk-heterojunction thin-film organic solar cells was found to have a significant impact on their optical and electrical properties the polymer films. Our experimental data demonstrated that the concentration of Ni/Ag NCs played a crucial role on the performance of the solar cells. The highest recorded PCE in this investigation is 6.2% achieved at a 2% weight concentration of Ni/Ag NCs. This is a very good increment in PCE given the nature of the polymer blends used in this investigation. The improvement in solar cell performance is attributed to Ni/Ag NCs local surface plasmon resonance effect that influences both light trapping and charge transport processes. The findings of this study provide valuable insights for future research in the field of organic solar cells and the design of more efficient and cost-effective solar cell devices. Further studies are required to optimize the concentration of Ni/Ag NCs by employing the NCs in different functional layers of the solar cell. It is to be noted that this experiment was conducted under ambient laboratory conditions which an important development in the realization of TFOSC. Finally, the Ag/Ni is environmentally stable and suitable for roll to roll large-scale device fabrication.
Data and code availability
All data are included in the manuscript. If necessary, data can be available upon request from authors.
References
Hoven CV, Dang XD, Coffin RC, Peet J, Nguyen TQ, Bazan GC (2010) Improved performance of polymer bulk heterojunction solar cells through the reduction of phase separation via solvent additives. Adv Mater 22(8):E63–E66
Brabec CJ, Durrant JR (2008) Solution-processed organic solar cells. MRS Bull 33(7):670–675
Coakley KM, McGehee MD (2004) Conjugated polymer photovoltaic cells. Chem Mater 16(23):4533–4542
Scharber MC, Mühlbacher D, Koppe M, Denk P, Waldauf C, Heeger AJ, Brabec CJ (2006) Design rules for donors in bulk-heterojunction solar cells-Towards 10% energy-conversion efficiency. Adv Mater 18(6):789–794
Dlamini MW, Mola GT (2019) Near-field enhanced performance of organic photovoltaic cells. Phys B Cond Matter 5(52):78–83
Fleetham T, Choi JY, Choi HW, Alford T, Jeong DS, Lee TS, Lee WS, Lee KS, Li J, Kim I (2015) Photocurrent enhancements of organic solar cells by altering dewetting of plasmonic Ag nanoparticles. Sci Rep 5(1):1–9
Oseni SO, Mola GT (2019) Bimetallic nanocomposites and the performance of inverted organic solar cell. Compos Part B 172:660–665
Hamed MSG, Adedeji MA, Zhang Y, Mola GT (2020) Silver sulphide nano-particles enhanced photo-current in polymer solar cells. Appl Phys A Mater Sci Semicond Process 126:126–207
Jo J, Na SI, Kim SS, Lee TW, Chung Y, Kang SJ, Vak D, Kim DY (2009) Three-dimensional bulk heterojunction morphology for achieving high internal quantum efficiency in polymer solar cells. Adv Funct Mater 19(15):2398–2406
Ye L, Zhang S, Ma W, Fan B, Guo X, Huang Y, Ade H, Hou J (2012) From binary to ternary solvent: morphology fine-tuning of D/A blends in PDPP3T-based polymer solar cells. Adv Mater 24(47):6335–6341
Wang J, Jia S, Cao Y, Wang W, Yu P (2018) Design principles for nanoparticle plasmon-enhanced organic solar cells. Nanoscale Res Lett 13(1):1–6
Kalfagiannis N, Karagiannidis PG, Pitsalidis C, Panagiotopoulos NT, Gravalidis C, Kassavetis S, Patsalas P, Logothetidis S (2012) Plasmonic silver nanoparticles for improved organic solar cells. Solar Energy Mater Solar Cells 104:165–174
Mandal P, Sharma S (2016) Progress in plasmonic solar cell efficiency improvement: a status review. Renew Sustain Energy Rev 65:537–552
Enrichi F, Quandt A, Righini GC (2018) Plasmonic enhanced solar cells: summary of possible strategies and recent results. Renew Sustain Energy Rev 82:2433–2439
Lee JM, Kim SO (2016) Enhancing organic solar cells with plasmonic nanomaterials. Chem Nano Mat 2(1):19–27
Tang M, Sun B, Zhou D, Gu Z, Chen K, Guo J, Feng L, Zhou Y (2016) Broadband plasmonic Cu-Au bimetallic nanoparticles for organic bulk heterojunction solar cells. Organic Electron 38:213–221
Hamed MS, Mola GT (2019) Copper sulphide as a mechanism to improve energy harvesting in thin film solar cells. J Alloys Compod 802:252–258
Wang CC, Choy WC, Duan C, Fung DD, Wei EI, Xie FX, Huang F, Cao Y (2012) Optical and electrical effects of gold nanoparticles in the active layer of polymer solar cells. J Mater Chem 22(3):1206–1211
Hamdan KS, Abdullah SM, Sulaiman K, Zakaria R (2014) Effects of silver nanoparticles towards the efficiency of organic solar cells. Appl Phys A 115:63–68
Kacus H, Biber M, Aydogan S (2020) Role of the Au and Ag nanoparticles on organic solar cells based on P3HT: PCBM active layer. Appl Phys A 126:1–9
Stratakis E, Kymakis E (2013) Nanoparticle-based plasmonic organic photovoltaic devices. Mater Today 16(4):133–146
Zhong Y, Malagari SD, Hamilton T, Wasserman D (2015) Review of mid-infrared plasmonic materials. J Nanophot 9(1):093791–093791
Holliday S, Ashraf RS, Wadsworth A, Baran D, Yousaf SA, Nielsen CB, Tan CH, Dimitrov SD, Shang Z, Gasparini N, Alamoudi M (2016) High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nature Commun 7(1):1–11
Cheng CE, Pei Z, Hsu CC, Chang CS, Chien FSS (2014) Hole transit in P3HT: PCBM solar cells with embedded gold nanoparticles. Solar Energy Mater Solar Cells 121:80–84
Xie FX, Choy WC, Wang CC, Sha WE, Fung DD (2011) Improving the efficiency of polymer solar cells by incorporating gold nanoparticles into all polymer layers. Appl Phys Lett 99(15):2145
Gao H, Meng J, Sun J, Deng J (2020) Enhanced performance of polymer solar cells based on P3HT: PCBM via incorporating Au nanoparticles prepared by the micellar method. J Mater Sci Mater Electron 31(13):10760–10767
Gollu SR, Sharma R, Srinivas G, Kundu S, Gupta D (2014) Effects of incorporation of copper sulfide nanocrystals on the performance of P3HT: PCBM based inverted solar cells. Organic Electron 15(10):2518–2525
Zhao J, Nguyen SC, Ye R, Ye B, Weller H, Somorjai GA, Alivisatos AP, Toste FD (2017) A comparison of photocatalytic activities of gold nanoparticles following plasmonic and interband excitation and a strategy for harnessing interband hot carriers for solution phase photocatalysis. ACS Central Sci 3(5):482–488
Barnes WL, Dereux A, Ebbesen TW (2003) Surface plasmon subwavelength optics. Nature 424(6950):824–830
Bohren CF, Huffman DR (1983) Absorption and scattering of light by small particles. Wiley, New York, p 26
Wang L, Hasanzadeh Kafshgari M, Meunier M (2020) Optical properties and applications of plasmonic-metal nanoparticles. Adv Funct Mater 30(51):2005400
Olson J, Dominguez-Medina S, Hoggard A, Wang LY, Chang WS, Link S (2015) Optical characterization of single plasmonic nanoparticles. Chem Soc Rev 44(1):40–57
Espinosa A, Reguera J, Curcio A, Muñoz-Noval Á, Kuttner C, Van de Walle A, Liz-Marzán LM, Wilhelm C (2020) Janus magnetic-plasmonic nanoparticles for magnetically guided and thermally activated cancer therapy. Small 16(11):1904960
Khurana K, Jaggi N (2021) Localized surface plasmonic properties of Au and Ag nanoparticles for sensors: a review. Plasmonics 16(4):981–999
Murray WA, Barnes WL (2007) Plasmonic materials. Adv Mater 19(22):3771–3782
Ahmed AY, Ike JN, Hamed MS, Mola GT (2023) Silver decorated magnesium doped photoactive layer for improved collection of photo-generated current in polymer solar cell. J Appl Polym Sci 140(14):e53697
Bhatia P, Verma SS, Sinha MM (2020) Tunable optical properties of Ni-Ag and Ni-Au nanoparticles in magneto-plasmonic nanostructures. Opt Quant Electron 52(11):1–12
Makuła P, Pacia M, Macyk W (2018) How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra. J Phys Chem Lett 9(23):6814–6817
Aziz SB, Abdullah OG, Rasheed MA (2017) A novel polymer composite with a small optical band gap: new approaches for photonics and optoelectronics. J Appl Polym Sci 134(21):5
Mbuyise XG, Mola GT (2020) Polycrystal metals nano-composite assisted photons harvesting in thin film organic solar cell. Solar Energy 208:930–936
Owen EA, Roberts EW (1939) XXIX. Factors affecting the limit of solubility of elements in copper and silver. London Edinb Dublin Philosoph Magaz J Sci 27(182):294–327
Wang X, Shi L, Zhang J, Cheng J, Wang X (2018) In situ formation of surface-functionalized ionic calcium carbonate nanoparticles with liquid-like behaviours and their electrical properties. Royal Soc Open Sci 5(1):170732
Mola GT, Mthethwa MC, Hamed MS, Adedeji MA, Mbuyise XG, Kumar A, Sharma G, Zang Y (2021) Local surface plasmon resonance assisted energy harvesting in thin film organic solar cells. J Alloys Compod 856:158172
Gaudry M, Cottancin E, Pellarin M, Lermé J, Arnaud L, Huntzinger JR, Vialle JL, Broyer M, Rousset JL, Treilleux M, Mélinon P (2003) Size and composition dependence in the optical properties of mixed (transition metal/noble metal) embedded clusters. Phys Rev B 67(15):155409
Hamed MS, Ahmed AY, Mola GT (2023) Suppressing charge recombination in disordered polymers blend medium. J Phys D Appl Phys 56(40):405101
Qi B, Wang J (2013) Fill factor in organic solar cells. Phys Chem Chem Phys 15(23):8972–8982
Dhass AD, Natarajan E, Ponnusamy L (2012) Influence of shunt resistance on the performance of solar photovoltaic cell. In: 2012 International conference on emerging trends in electrical engineering and energy management (ICETEEEM) (pp. 382-386). IEEE
Proctor CM, Nguyen TQ (2015) Effect of leakage current and shunt resistance on the light intensity dependence of organic solar cells. Appl Phys Lett 106(8):23–41
Tessema G (2012) Charge transport across bulk heterojunction organic thin film. Appl Phys A 106:53–57
Nardes AM, Kemerink M, De Kok MM, Vinken E, Maturova K, Janssen RAJ (2008) Conductivity, work function, and environmental stability of PEDOT: PSS thin films treated with sorbitol. Organic Electron 9(5):727–734
Brutting W, Berleb S, Mückl AG (2001) Device physics of organic light-emitting diodes based on molecular materials. Organic Electron 2(1):1–36
Vanlaeke P, Vanhoyland G, Aernouts T, Cheyns D, Deibel C, Manca J, Heremans P, Poortmans J (2006) Polythiophene based bulk heterojunction solar cells: morphology and its implications. Thin Solid Films 511:358–361
Sha WE, Li X, Choy WC (2014) Breaking the space charge limit in organic solar cells by a novel plasmonic-electrical concept. Sci Rep 4(1):1–10
Bartelt JA, Lam D, Burke TM, Sweetnam SM, McGehee MD (2015) Charge-carrier mobility requirements for bulk heterojunction solar cells with high fill factor and external quantum efficiency> 90%. Adv Energy Mater 5(15):1500577
Omani M, Fallah H, Choy KL, Abdi-Jalebi M (2021) Impact of hybrid plasmonic nanoparticles on the charge carrier mobility of P3HT: PCBM polymer solar cells. Sci Rep 11(1):19774
Mola GT, Mthethwa MC, Hamed MS, Adedeji MA, Mbuyise XG, Kumar A, Sharma G, Zang Y (2021) Local surface plasmon resonance assisted energy harvesting in thin film organic solar cells. J Alloys Compd 856:158172
Acknowledgements
This work is supported by the National Research Foundation (NRF) (Grant Nos. 93562, 113835, and 132786), South Africa. The authors also appreciate the members of staff in the Microscopy and Microanalysis Unit (MMU) at UKZN, for several SEM and TEM measurements.
Funding
Open access funding provided by University of KwaZulu-Natal.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally at all stage of the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Dale Huber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, A.Y.A., Hamed, M.S.G., Ike, J.N. et al. Nickel-doped silver nanoclusters as a mechanism to capture photons. J Mater Sci 59, 8942–8955 (2024). https://doi.org/10.1007/s10853-024-09690-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09690-6