Abstract
A new bifunctional nanomaterial, [SBCu(II)Hyd]-MWCNTs, exhibiting exotic electrical and magnetic properties has been synthesized via chemical modification of MWCNT-COOH. Double probe DC electrical conductivity, CV and EIS studies show better conductivity of the material than that of MWCNT-COOH. With higher saturation and remanent magnetization, as well as coercivity, [SBCu(II)Hyd]-MWCNTs showed better ferromagnetic characteristics. Mott–Schottky electrochemical analysis was carried out to explore capacitive and dielectric properties. The enhancement in electrical conductivity of [SBCu(II)Hyd]-MWCNTs is also confirmed by optical and electrochemical band gaps studies. Subsequently, this material has been utilized to fabricate an electrochemical sensor by coating it over glassy carbon electrode for the determination of glucose. The corresponding sensitivity and limit of detection values are calculated to be 1.1 µA µM−1 cm−2 and 0.09 µM, respectively.
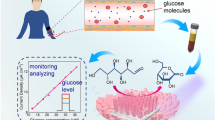
Graphical Abstract











Similar content being viewed by others

Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to legal or ethical reasons.
References
Dwivedi N, Kumar S, Carey JD, Dhand C (2015) Functional nanomaterials for electronics, optoelectronics, and bioelectronics. J Nanomater 2015:1–1
Wongkaew N, Simsek M, Griesche C, Baeumner AJ (2018) Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: recent progress, applications, and future perspective. Chem Rev 119(1):120–194
Xue X, Wang F, Liu X (2011) Emerging functional nanomaterials for therapeutics. J Mater Chem 21(35):13107–13127
Zhu C, Liu T, Qian F, Chen W, Chandrasekaran S, Yao B, Song Y, Duoss EB, Kuntz JD, Spadaccini CM, Worsley MA (2017) 3D printed functional nanomaterials for electrochemical energy storage. Nano Today 15:107–120
Zaera F (2013) Nanostructured materials for applications in heterogeneous catalysis. Chem Soc Rev 42(7):2746–2762
Yin Y, Talapin D (2013) The chemistry of functional nanomaterials. Chem Soc Rev 42(7):2484–2487
Ghomi LS, Behzad M, Tarahhomi A, Arab A (2017) Crystal structures, DFT calculations, and Hirshfeld surface analyses of two new copper (II) and nickel (II) Schiff base complexes derived from meso-1, 2-diphenyl-1, 2-ethylenediamine. J Mol Struct 1150:214–226
Canali L, Sherrington DC (1999) Utilisation of homogeneous and supported chiral metal (salen) complexes in asymmetric catalysis. Chem Soc Rev 28(2):85–93
Jones RD, Summerville DA, Basolo F (1979) Synthetic oxygen carriers related to biological systems. Chem Rev 79(2):139–179
Casas JS, Couce MD, Sordo J (2012) Coordination chemistry of vitamin B6 and derivatives: a structural overview. Coord Chem Rev 256(23–24):3036–3062
Oiye ÉN, Ribeiro MF, Katayama JM, Tadini MC, Balbino MA, Eleotério IC, Magalhães J, Castro AS, Silva RS, da Júnior CJW, Dockal ER (2019) Electrochemical sensors containing Schiff bases and their transition metal complexes to detect analytes of forensic, pharmaceutical and environmental interest. A review. Crit Rev Anal Chem 49(6):488–509
More MS, Joshi PG, Mishra YK, Khanna PK (2019) Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: a review. Mat Today Chem 14:100195
Gebreyesus ST, Khan MA (2015) An overview on metal complexes of selected schiff-bases with their electrochemical and sensor aspects. J Chem Chem Sci 5(1):19–27
Garoufis A, Hadjikakou SK, Hadjiliadis NJ (2009) Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord Chem Rev 253(9–10):1384–1397
Jeevadason AW, Murugavel KK, Neelakantan MA (2014) Review on Schiff bases and their metal complexes as organic photovoltaic materials. Renew Sustain Energ Rev 36:220–227
Zhang J, Xu L, Wong WY (2018) Energy materials based on metal Schiff base complexes. Coord Chem Rev 355:180–198
Miyasaka H, Saitoh A, Abe S (2007) Magnetic assemblies based on Mn (III) salen analogues. Coord Chem Rev 251(21–24):2622–2664
Kumar KS, Bayeh Y, Gebretsadik T, Elemo F, Gebrezgiabher M, Thomas M, Ruben M (2019) Spin-crossover in iron (II)-Schiff base complexes. Dalton Trans 48(41):15321–15337
Wang L, Jiao S, Zhang W, Liu Y, Yu G (2013) Synthesis, structure, optoelectronic properties of novel zinc Schiff-base complexes. Chi Sci Bull 58:2733–2740
Al-Hazmi GA, El-Metwally N (2017) A series of nickel (II) complexes derived from hydrazide derivatives, electrochemical, thermal and spectral studies. Arab J Chem 10:S1003–S1013
Borthakur R, Kumar A, De AK, Lal RA (2019) Synthesis, characterization and electrochemical properties of copper (II) complexes derived from succinoyldihydrazine Schiff base ligands. Arab J Chem 12(8):2192–2205
Dhanakodi P, Jayandran M, Balasubramanian V (2018) Syntheses and characterization of complexes of copper (II) with Schiff-base ligands derived from 2, 6-diacetylpyridine: spectroscopic, thermal behavior, magnetic moment andphotoluminescent studies. J Mater Sci Mater Electron 29:7526–7530
Guo Y, Hu X, Zhang X, Pu X, Wang Y (2019) The synthesis of a Cu (ii) Schiff base complex using a bidentate N2O2 donor ligand: crystal structure, photophysical properties, and antibacterial activities. RSC Adv 9(71):41737–41744
Sridara T, Upan J, Saianand G, Tuantranont A, Karuwan C, Jakmunee J (2020) Non-enzymatic amperometric glucose sensor based on carbon nanodots and copper oxide nanocomposites electrode. Sensors 20(3):808
Kannan P, Maiyalagan T, Marsili E, Ghosh S, Guo L, Huang Y, Rather JA, Thiruppathi D, Niedziolka-Jönsson J, Jönsson-Niedziolka M (2017) Highly active 3-dimensional cobalt oxide nanostructures on the flexible carbon substrates for enzymeless glucose sensing. Analyst 142(22):4299–4307
Haghparas Z, Kordrostami Z, Sorouri M, Rajabzadeh M, Khalifeh R (2020) Fabrication of non-enzymatic electrochemical glucose sensor based on nano-copper oxide micro hollow-spheres. Biotechnol Bioprocess Eng 25:528–535
Jiang D, Liu Q, Wang K, Qian J, Dong X, Yang Z, Du X, Qiu B (2014) Enhanced non-enzymatic glucose sensing based on copper nanoparticles decorated nitrogen-doped graphene. Biosens Bioelectron 54:273–278
Baingane A, Narayanan JS, Slaughter G (2018) Sensitive electrochemical detection of glucose via a hybrid self-powered biosensing system. Sens Bio-sens Res 20:41–46
Dai Z, Yang A, Bao X, Yang R (2019) Facile non-enzymatic electrochemical sensing for glucose based on Cu2O–BSA nanoparticles modified GCE. Sensors 19(12):2824
Perušković DS, Stevanović NR, Kovačević GN, Stanković DM, Lolić AĐ, Baošić RM (2020) Application of N, N’-Bis (acetylacetonato) propylenediimine copper (II) complex as mediator for glucose biosensor. Chem Sel 5(5):1671–1675
Batchelor-McAuley C, Wildgoose GG, Compton RG, Shao L, Green ML (2008) Copper oxide nanoparticle impurities are responsible for the electroanalytical detection of glucose seen using multiwalled carbon nanotubes. Sens Act B Chem 132(1):356–360
Hu C, Hu S (2009) Carbon nanotube-based electrochemical sensors: principles and applications in biomedical systems. J Sens. https://doi.org/10.1155/2009/187615
Sonkar PK, Ganesan V, John SA, Yadav DK, Gupta R (2016) Non-enzymatic electrochemical sensing platform based on metal complex immobilized carbon nanotubes for glucose determination. RSC Adv 6(108):107094–107103
Rezaeinasab M, Benvidi A, Tezerjani MD, Jahanbani S, Kianfar AH, Sedighipoor M (2017) An electrochemical sensor based on Ni (II) complex and multi wall carbon nano tubes platform for determination of glucose in real samples. Electroanalysis 29(2):423–432
Hasanzadeh M, Hasanzadeh Z, Alizadeh S, Sayadi M, Nezhad NM, Sabzi E, R., & Ahmadi, S. (2020) Copper-nickel oxide nanofilm modified electrode for non-enzymatic determination of glucose. J Electrochem Sci Eng 10(3):245–255
Yang Z, Feng J, Qiao J, Yan Y, Yu Q, Sun K (2012) Copper oxide nanoleaves decorated multi-walled carbon nanotube as platform for glucose sensing. Anal Methods 4(7):1924–1926
Figiela M, Wysokowski M, Galinski M, Jesionowski T, Stepniak I (2018) Synthesis and characterization of novel copper oxide-chitosan nanocomposites for non-enzymatic glucose sensing. Sens Act B Chem 272:296–307
Bernasconi R, Mangogna A, Magagnin L (2018) Low cost inkjet fabrication of glucose electrochemical sensors based on copper oxide. J Electrochem Soc 65(8):B3176
Maaoui H, Teodoresu F, Wang Q, Pan GH, Addad A, Chtourou R, Szunerits S, Boukherroub R (2016) Non-enzymatic glucose sensing using carbon quantum dots decorated with copper oxide nanoparticles. Sensors 16(10):1720
Gupta R, Singh B (2020) Chemical modification of carboxylated MWCNTs for enhanced electrical conducting and magnetic properties. Mat Sci Eng B 262:114730
Bazarganipour M, Salavati-Niasari M (2016) Synthesis, characterization and chemical binding of a Ni (II) Schiff base complex on functionalized MWNTs; Catalytic oxidation of cyclohexene with molecular oxygen. Chem Eng J 286:259–265
Veisi H, Azadbakht R, Saeidifar F, Abdi MR (2017) Schiff base-functionalized multi walled carbon nanotubes to immobilization of palladium nanoparticles as heterogeneous and recyclable nanocatalyst for Suzuki reaction in aqueous media under mild conditions. Catal Lett 147:976–986
Singh MS, Tawade K (2000) Synthesis and characterization of some new organotin (Iv) complexes of a Schiff base derived from salicylaldehyde and hydrazine hydrate. Synth React Inorg Mat Org Chem 30(6):1015–1022
Dikio CW, Ejidike IP, Mtunzi FM, Klink MJ, Dikio ED (2017) Hydrazide Schiff bases of acetylacetonate metal complexes: synthesis, spectroscopic and biological studies. Int J Pharm Pharm Sci 12:257–267
Lekshmy RK, Thara GS (2014) Synthesis and characterization of copper complexes of Schiff base derived from isatin and salicylic hydrazide. In: AIP Conference Proceedings, American Institute of Physics 1620(1): 230-234
Kolcu F, Kaya İ (2017) Synthesis, characterization and photovoltaic studies of oligo (acriflavine) via chemical oxidative polymerization. RSC Adv 7(15):8973–8984
Gupta M, Pal SK (2016) Triphenylene-based room-temperature discotic liquid crystals: a new class of blue-light-emitting materials with long-range columnar self-assembly. Langmuir 32(4):1120–1126
Zhang G, Yang D, Sacher E (2007) X-ray photoelectron spectroscopic analysis of Pt nanoparticles on highly oriented pyrolytic graphite, using symmetric component line shapes. J Phy Chem C 111(2):565–570
Ourari A, Derafa W, Aggoun D (2015) A novel copper (II) complex with an unsymmetrical tridentate-Schiff base: synthesis, crystal structure, electrochemical, morphological and electrocatalytic behaviors toward electroreduction of alkyl and aryl halides. RSC Adv 5(101):82894–82905
Reddy GR, Balasubramanian S, Chennakesavulu K (2014) Zeolite encapsulated Ni (ii) and Cu (ii) complexes with tetradentate N2O2 Schiff base ligand: catalytic activity towards oxidation of benzhydrol and degradation of rhodamine-B. J Mater Chem A 2(37):15598–15610
Bora SJ, Chetia B (2019) Synthesis of ynones at room temperature catalyzed by copper chloride cryptand complex under solvent free conditions. Heliyon 5(7):e02000
Gayen FR, Ali AA, Bora D, Roy S, Saha S, Saikia L, Goswamee RL, Saha B (2020) A ferrocene functionalized Schiff base containing Cu (II) complex: synthesis, characterization and parts-per-million level catalysis for azide alkyne cycloaddition. Dalton Trans 49(20):6578–6586
Vafakish B, Wilson LD (2020) Cu (II) Ion adsorption by Aniline grafted Chitosan and its responsive fluorescence properties. Molecules 25(5):1052
Bokobza LA, Zhang J (2012) Raman spectroscopic characterization of multiwall carbon nanotubes and of composites. Express Polym Lett 6(7):601
Park OK, Lee S, Joh HI, Kim JK, Kang PH, Lee JH, Ku BC (2012) Effect of functional groups of carbon nanotubes on the cyclization mechanism of polyacrylonitrile (PAN). Polymer 53(11):2168–2174
Salavati-Niasari M, Davar F, Bazarganipour M (2010) Synthesis, characterization and catalytic oxidation of para-xylene by a manganese (III) Schiff base complex on functionalized multi-wall carbon nanotubes (MWNTs). Dalton Trans 39(31):7330–7337
Wang F, Arai S, Endo M (2004) Metallization of multi-walled carbon nanotubes with copper by an electroless deposition process. Electrochem Commun 6(10):1042
Theivasanthi T, Alagar M (2010) X-ray diffraction studies of copper nanopowder. arXiv preprintarXiv:1003.6068 Mar 31.https://doi.org/10.48550/arXiv.1003.6068
Zhao J, Xie Y, Guan D, Hua H, Zhong R, Qin Y, Fang J, Liu H, Chen J (2015) BaFe12O19-chitosan Schiff-base Ag (I) complexes embedded in carbon nanotube networks for high-performance electromagnetic materials. Sci Rep 5(1):1–1
Abdalla AM, Sahu RP, Wallar CJ, Chen R, Zhitomirsky I, Puri IK (2017) Nickel oxide nanotube synthesis using multiwalled carbon nanotubes as sacrificial templates for supercapacitor application. Nanotechnology 28(7):075603
Morales I, Costo R, Mille N, Da Silva GB, Carrey J, Hernando A, De la Presa P (2018) High frequency hysteresis losses on γ-Fe2O3 and Fe3O4: Susceptibility as a magnetic stamp for chain formation. Nanomaterials 8(12):970
Akhtar AJ, Gupta A, Chakravorty D, Saha SK (2013) Antiferroquadrupolar ordering in Fe intercalated few layers graphene. AIP Adv 3(7):072124
Petrovský E, Kapička A (2006) On determination of the Curie point from thermomagnetic curves. J Geophys Res Solid Earth. https://doi.org/10.1029/2006JB004507
Gupta R, Singh B (2019) Enhancement of electrical conductivity and magnetic properties of bimetallic Schiff base complex on grafting to MWCNTs. J Mater Sci Mater Electron 30:11888–11906
Jahan M, Bao Q, Loh KP (2012) Electrocatalytically active graphene−porphyrin MOF composite for oxygen reduction reaction. J Am Chem Soc 134:6707–6713
Parida K, Dehury SK, Choudhary RN (2016) Structural, electrical and magneto-electric characteristics of BiMgFeCeO6 ceramics. Phys Lett A 380(48):4083–4091
Nan M, Bi Y, Xue H, Xue S, Long H, Pu L, Fu G (2019) Rapid determination of ochratoxin A in grape and its commodities based on a label-free impedimetric aptasensor constructed by layer-by-layer self-assembly. Toxins 11(2):71
Ali GA, Yusoff MM, Shaaban ER, Chong KF (2017) High performance MnO2 nanoflower supercapacitor electrode by electrochemical recycling of spent batteries. Ceram Inter 43(11):8440–8448
Datta J, Das M, Dey A, Halder S, Ray SS (2017) Network analysis of semiconducting Zn1-xCdxS based photosensitive device using impedance spectroscopy and current-voltage measurement. Appl Surf Sci 420:566–578
Víllora EG, Shimamura K, Yoshikawa Y, Ujiie T, Aoki K (2008) Electrical conductivity and carrier concentration control in β-Ga2O3 by Si doping. Appl Phys Lett 92(20):202120
Rashad MM, Hassan AM, Nassar AM, Ibrahim NM, Mourtada A (2014) A new nano-structured Ni (II) Schiff base complex: synthesis, characterization, optical band gaps, and biological activity. Appl Phys A 117:877–890
Yunus K, Mutlu H, Gazi İ (2010) Uv-vis spectra and fluorescence properties of two iminooxime ligands and their metal complexes: optical band gaps. Gazi Univ J Sci 23(1):13–18
Guy OJ, Walker KAD (2016) Graphene functionalization for biosensor applications. Biotechnol 2016:85–141
Deroco PB, de FátimaGiarolaJ JDW, Lorga GA, Kubota LT (2020) Paper based electrochemical sensing devices. Compr Anal Chem 89:91–137
Gupta R, Rastogi PK, Ganesan V, Yadav DK, Sonkar PK (2017) Gold nanoparticles decorated mesoporous silica microspheres: a proficient electrochemical sensing scaffold for hydrazine and nitrobenzene. Sens Act B Chem 239:970–978
Zhang Y, Bo X, Nsabimana A, Han C, Li M, Guo L (2015) Electrocatalytically active cobalt-based metal–organic framework with incorporated macroporous carbon composite for electrochemical applications. J Mater Chem A 3(2):732–738
Yadav M, Ganesan V, Maiti B, Gupta R, Sonkar PK, Yadav DK, Walcarius A (2019) Sensitive determination of acetaminophen in the presence of dopamine and pyridoxine facilitated by their extent of interaction with single-walled carbon nanotubes. Electroanalysis 31(12):2472–2479
Bruen D, Delaney C, Florea L, Diamond D (2017) Glucose sensing for diabetes monitoring: recent developments. Sensors 17:1866
Macaya DJ, Nikolou M, Takamatsu S, Mabeck JT, Owens RM, Malliaras GG (2007) Simple glucose sensors with micromolar sensitivity based on organic electrochemical transistors. Sens Act B Chem 123(1):374–378
Acknowledgements
I would like to delightedly acknowledge Department of Science and Technology (DST Scheme No. SR/NM/NS-1212/2013), New Delhi, India, for giving me a research fellowship, which served as financial support, as well as the VSM facilities to do my research. Incentive fund from IOE, B. H. U, (IOE/Dev. Scheme No. 6031), Ministry of Human Resource and Development is also acknowledged. I am also thankful to Late Prof. O. N. Srivastava, Department of Physics for providing TEM facility. Central Instrumentation Facility of I.I.T. B.H.U is acknowledged for temperature dependent magnetization studies. Advanced Centre for Material Sciences, IIT Kanpur is highly acknowledged for XPS analysis of synthesized materials.
Author information
Authors and Affiliations
Contributions
RG took part in conceptualization, experimental setup, and preparation of materials, formal analysis, investigation and writing original draft. Formal analysis, investigation and writing—review & editing were performed by MY. The selectivity, reproducibility and stability studies to verify the performance of proposed glucose sensor have been investigated by SS. The electrochemical studies were supervised by VG. Validation, investigation, resources, writing- review & editing, supervision and project administration were achieved by BS.
Corresponding author
Ethics declarations
Conflict of interest
The Authors have no conflicts of interest to declare.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, R., Yadav, M., Singh, S. et al. Design and development of a multiwalled carbon nanotubes-based copper (II) Schiff base complex for the facile non-enzymatic electrochemical sensing of glucose. J Mater Sci 58, 12312–12330 (2023). https://doi.org/10.1007/s10853-023-08774-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08774-z