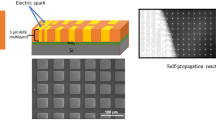
Abstract
Reactive multilayer systems consisting of alternating nanoscale Al and Ni layers are applicable in joining, various pyrotechnic applications and thermal batteries. Since diffusion based high-temperature synthesis occurs without the presence of air, efforts have focused on investigating the understanding of the fundamental reaction processes and characteristics. The aim of this study is to expose the reactive multilayers to extrinsic induced tensile stress so that the self-propagating synthesis can proceed under these conditions. Further, the properties during and after the reaction will be investigated. Multilayers deposited by sputtering on Kapton® substrates with different bilayer– and total thicknesses as well as commercial Nanofoils® with thicknesses of 40 µm and 60 µm were used as samples. The investigations focused on the propagation velocity measured with a high-speed camera, the temperature regime determined with a high-speed pyrometer, and the formed phases after the synthesis examined via X-ray diffraction. The gained results of this study reveal important insights for the application of the reactive Al/Ni multilayer system in terms of stability or reliability related to propagation front velocity, maximum temperature and formed phases under induced external tensile stresses.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alternating nanolayered multilayers of Al and Ni belong to the so-called reactive multilayer systems (RMS), which are characterized by a self-sustained high-temperature synthesis (SHS). The Al/Ni system in particular is one of the most investigated systems in the group of reactive multilayers. Numerous studies have been carried out with regard to joining [1,2,3], simulations or numerical investigations [4,5,6,7] and microstructural analysis [8,9,10,11,12,13,14]. Only few studies have focused on the impact of stress and its influence on the SHS before, during or after the reaction. Primarily the investigations were focused on internal stresses in these types of multilayers [15,16,17]. Reiss et al. conducted a study in which they demonstrated the superplastic deformation and the possibility of shaping of Nb/Si RMS, facilitating the estimation of the biaxial stress state, plastic strain and strain rate [18]. The studies conducted by Azis et al. [19, 20] have shown that the diffusivity of materials is influenced by the presence of stresses, thereby affecting the overall reaction, which is predominantly diffusion-driven. These results underline the crucial role of stresses in relation to diffusion in determining the kinetics of the reaction. However, the issue of stress is a permanent topic especially in thin films and functional coatings due to the fact that stresses, internal or external, can affect, e.g. the performance, reliability and durability of a system or the materials by themselves [21, 22]. In this context, intrinsic stresses are those that originate from the material itself, which are also named residual stresses. Especially in the field of thin films, this is caused by complex dependencies of various factors, such as atomic mobility, growth rate, film microstructure and morphology [21,22,23]. Here, external stresses are stresses that are additionally induced in the system by an external event and are superimposed on the residual stresses. This can be, for example, an external mechanical load or strain.
Primarily during the application of RMS external induced stresses could appear. Based on previous studies it is pointed out that stresses beside influencing the diffusion rates as well could influence the chemical composition at the interphase boundaries and due to this the overall kinetics of the system [24,25,26]. Furthermore, there is the possibility of more than one stable state for a two-phase alloy by changing the phase equilibria due to internal stresses [27]. However, there is still a lack of knowledge regarding the influence of externally induced stress on the SHS of RMS. The motivation of this work is to contribute a part of this extensive topic for the Al/Ni RMS.
Therefore, in this study alternated nanolayered Al/Ni multilayer with different bilayer—and total thicknesses was deposited by sputtering on Kapton® due to its relatively low thermal conductivity (0.12 Wm−1 K−1) and pronounced elastic properties (ultimate elongation: 70-80%). Additionally, freestanding commercial Nanofoils® with thicknesses of 40 µm and 60 µm were used as reference samples during the experiments in order to investigate possible scaling effects. The aim of investigation is to induce extrinsic tensile stresses to the specimens and examine the velocity of the propagation using a high-speed camera as well as the temperature regime using a high-speed pyrometer during the synthesis. Further, the evolved phases after the reaction were analyzed using X-ray diffraction (XRD).
Materials and methods
Materials
The used samples consisting of alternating Al and Ni nanolayers were deposited by direct current (DC) magnetron sputtering in a cluster deposition system (Von Ardenne CS400). In order to obtain high qualitative nanolayers with a minority of contaminations two targets of pure metals with purities of Al – 5N (FHR) and Ni – 3N8 (FHR) were used. As sputtering gas Ar (6N) with a gas flow of 30 sccm was used which results in a process pressure of 5 × 10−3 mbar. The sputtering power was set to 200 W for both targets, respectively, resulting in a sputtering rate of 0.33 nm s−1 for Al and 0.35 nm s−1 for Ni. In order to obtain an average stoichiometric ratio of A50Ni50 the layer thickness was set to a ratio of Al/Ni = 3:2. As the first layer to the substrate, Al was chosen, consequently, Ni becomes the top layer in the alternating layer design to minimize surface oxidation. Due to low thermal conductivity (0.12 Wm−1 K−1) Kapton® foils with thicknesses of 50 µm and 125 µm were used as substrate during the deposition and the experiments. Additionally, the properties of Kapton® in terms of elasticity and thermal stability were ideally suited for the strain experiments carried out in combination with the SHS in Al/Ni nanolayered films. The schematic structure of the self-produced samples could be seen in Fig. 1. Different combinations of bilayer (Λ)—and total thickness (thtotal) and number of bilayers (n), see Table 1, were produced for the investigations.
In addition to the self-produced samples, freestanding commercial Nanofoils® with thicknesses of 40 µm and 60 µm were used for the investigations. It should be mentioned that these samples are covered on both sides with about 1 µm of brazing alloy Incusil™.
Methods
To determine the surface roughness of the substrates measurements with a laser scanning microscope (LSM) (Olympus LEXT OLS410) in an area of 649 µm × 649 µm was carried out. Samples in the “as deposited” state were investigated in the unreacted condition with XRD (Siemens D5000) to elucidate if a phase formation between Al and Ni already took place during the sputtering process. A gracing incidence (GI) geometry was used with an incidence angle of 5°, a step width of 0.02° and 1 s per step. The parameters of the Cu–Kα source (λ = 0.15418 nm) were set to 40 kV and 40 mA. Furthermore, scanning electron microscope (SEM)—pictures (Auriga 60 Zeiss) from the surface of the reactive multilayers were carried out. Cross-sectional views were done by focused ion beam (FIB) nano milling (Auriga 60 Zeiss) with a Ga source in order to analyze the single layer—and total thicknesses as well as the layer morphology.
In this work the focus is on extrinsically induced stresses with direction parallel to the interfaces of the Al/Ni multilayers. To apply a tensile strain the samples were clamped into a tensile machine (Hegewald & Peschke inspekt blue 20 kN), while both the substrate and the deposited layers were gripped by the sample holder. The schematic experimental setup could be seen in Fig. 2.
The traverse of the tensile machine was moved displacement-controlled at a speed of 2 mm min−1. After a preload of 0.1 N was applied to the specimen, the strain was determined from this point via the traverse distance traveled. An important point is to avoid cracks during the tensile test, otherwise the induced stresses will be relaxed and the experiment is failed. From our own preliminary investigations, it is known when the first cracks appear in the RMS under tensile load. In order to assess the effect of strain on the self-produced RMS and Nanofoils®, strain steps were set to 0, 0.5 and 1.0% for the self-produced RMS and 0, 0.1, 0.3, 0.5, 0.7 and 0.9% for the Nanofoils®. Once a certain strain step was reached, the SHS was initiated electrically with a laboratory power supply (PeakTech® 6080). Simultaneously the reaction was recorded with a high-speed camera (Photron FASTCAM SA-X2) with resolution set at 50,000 fps and a high-speed pyrometer (Kleiber series 840 + optic: 25-S1) with a resolution time of 10 µs. The measuring point of the pyrometer has a diameter of 400 µm. For all measurements the emission coefficient for the pyrometer was set to ε = 1. The measurements of reaction front velocity and temperature were examined for each strain on at least three samples.
To investigate the formed phases after the reaction GI-XRD with the aforementioned conditions was performed.
Results
Substrate
The surface roughness of the Kapton® substrates was estimated in an area of 649 µm x 649 µm. For each sample five measurements in vertical and horizontal directions, respectively, were carried out. The measured roughness for the 50 µm Kapton® is given as Ra = (0.014 ± 0.003) µm and the roughness of 125 µm Kapton® as Ra = (0.041 ± 0.002) µm.
Before reaction
The XRD patterns of the as-deposited specimens in Fig. 3 were evaluated in the area of 30°–100° (2θ) and show that no intermetallic phase between Al and Ni could be detected. Small shifts of the Al peaks to higher diffraction angles could be observed, in contrast to this there were only negligible shifts of the Ni peaks.
In Fig. 4 the cross-sectional and top view pictures are shown for Al/Ni multilayers deposited on 50 µm as well as on 125 µm thick Kapton® and 60 µm Nanofoils®. It should be noted, that a Pt–C layer was deposited on the surface to protect the RMS from the impact of the Ga ions during the nano milling process for the cross-sectional views. Additionally, the brazing alloy Incusil™ of the commercial Nanofoils® could be seen in Fig. 4f).
The top view reveals that multilayers deposited on the 50 µm thick Kapton® have much smaller surface structures compared to the samples on 125 µm thick Kapton®. In the cross-sectional views a similar effect could be seen according to the interface roughness. Additionally, defects in the Al/Ni multilayers deposited on 125 µm thick Kapton® could be mentioned. These could be assigned to the category of microcracks or hole-like defects [28].
During reaction
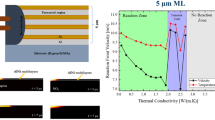
Figure 6 shows the velocity of the propagating reaction front from the self-propagating high-temperature synthesis (SHS) as a function of the applied extrinsic strain. Several pertinent issues can be addressed in this context, commencing with the comparison of the influence of the total thickness. As the total thickness of RMS increases, the speed of the reaction front also increases. This tendency becomes clear when the results of the samples ■–thtotal = 1 µm–Λ = 50 nm–50 µm Kapton®,  –thtotal = 2 µm–Λ = 50 nm–50 µm Kapton® and
–thtotal = 2 µm–Λ = 50 nm–50 µm Kapton® and  thtotal = 5 µm–Λ = 50 nm–50 µm Kapton® as well as the samples
thtotal = 5 µm–Λ = 50 nm–50 µm Kapton® as well as the samples  –thtotal = 2 µm–Λ = 50 nm–125 µm Kapton® and
–thtotal = 2 µm–Λ = 50 nm–125 µm Kapton® and  –thtotal = 5 µm–Λ = 50 nm–125 µm Kapton® are compared with each other.
–thtotal = 5 µm–Λ = 50 nm–125 µm Kapton® are compared with each other.
As a function of the induced tensile stress, slight differences of < 1.0 m s−1 in the reaction front velocity were observed for all self-produced specimens. The samples  –thtotal = 2 µm–Λ = 50 nm–50 µm Kapton® and
–thtotal = 2 µm–Λ = 50 nm–50 µm Kapton® and  –thtotal = 5 µm–Λ = 50 nm–50 µm Kapton® tended to increase, while the samples
–thtotal = 5 µm–Λ = 50 nm–50 µm Kapton® tended to increase, while the samples  –thtotal = 2 µm–Λ = 50 nm–125 µm Kapton® and
–thtotal = 2 µm–Λ = 50 nm–125 µm Kapton® and  –thtotal = 5 µm–Λ = 50 nm–125 µm Kapton® tended to decrease in reaction front velocity with higher strain.
–thtotal = 5 µm–Λ = 50 nm–125 µm Kapton® tended to decrease in reaction front velocity with higher strain.
The commercial Nanofoils® are characterized by a uniform velocity, which is merely subject to slight differences of ± 0.5 m s−1. For both the 40 µm and 60 µm thick films, no tendencies of the reaction front velocity in correlation with the applied strain of the films were evident. It must be taken into account at this point that the reaction takes place under the brazing layer present on these foils. A visual assessment of the speed is made by the visible melting of this layer. Minor deviations in the front speed could be masked by this.
Figure 6 shows an example of the temperature measurements that are representative for all the investigated samples. While propagating the sample without any applied strain, we observed the detachment of particles, leading to a discernible deviation in the cooling curve. Notably, the pyrometer signal was affected by the presence of these detached particles, contributing to the observed deviation. A very steep temperature increase could be seen when the reaction front reaches the pyrometer measuring spot, which is characteristic and similar for all samples. Furthermore, it can be observed that the maximum temperatures obtained from these samples remains constant despite the variation of the applied strain.
An overview to the measured maximum temperatures according to applied strain is presented in Fig. 7. All self-produced multilayer foils show similar maximum temperature values with a deviation of ± 30 K. The 40 µm Nanofoils® shows a larger deviation with respect to the applied strain but without a clear tendency. Similar to the determination of the reaction front velocity, the brazing layer present on the foil surface should be mentioned at this point, which is measured here.
After reaction
The results of the phase analysis are shown in Fig. 8 using sample with the parameters thtotal = 5 µm–Λ = 50 nm–50 µm Kapton® as a reference. According to different applied strains during the reaction no peak shifts as well as variation of peak ratios could be recognized in the reacted products. After the high-temperature synthesis, the samples in this study form the B2 phase AlNi (PDF 65–0431) which fits to the targeted ratio of Al50Ni50 during the deposition.
Discussion
XRD of as-deposited RMS
An explanation for the peak shifts of the Al peaks to higher diffraction angle in Fig. 3 could be due to the intermixing during the sputtering process at the interfaces. Furthermore, the activation energy for diffusion of Al in Ni (280 kJ mol−1) is higher than the reverse direction from Ni in Al (154 kJ mol−1) [29]. Although the peaks of Ni in the XRD diffractogram do not show any peak shift, see Fig. 3, the TEM studies of the interfaces by Ramos et al. [9] indicate that Al is also present in the Ni layers. However, the amount of Al in Ni lattice will be much lower than in the reverse case of Ni in Al lattice due to the larger activation energy of diffusion. Thus, this amount could be so small that only a nonsignificant influence is present during the XRD investigations and therefore no clear Ni peak shifts can be seen in the diffractogram. Additionally, a high intensity of the (111) lattice plane could be mentioned what is typical for face centered cubic metals like Al and Ni [30]. It should be noted that previous studies by Ramos et al., using XRD analysis, also failed to detect the presence of intermetallic phases in the as-deposited samples. However, in more detailed studies using high resolution transmission electron microscopy and fast Fourier transform, the evidence of intermetallic AlNi formation in as-deposited samples was observed, which means that it should have formed during the deposition process [9]. This means that the presence of intermetallic phases, especially at the interfaces, cannot be excluded in our studies.
Influence of extrinsic induced tensile stress to reaction front velocity
In Fig. 5 the effect was shown, that as the total thickness of RMS increases, the speed of the reaction front also increases when the high-temperature synthesis is propagating on a Kapton® substrate. This effect could not be observed for the freestanding commercial Nanofoils®. An explanation for this could be due to the influence of the substrate, more precise the thermal conductivity. Despite the relatively low thermal conductivity the substrate hinders the reaction by absorbing heat from the exothermic reaction by the phase building process which then leads to lower diffusion rates what is correlating to the reaction front velocity [22, 23, 31]. From this it can be concluded that the thicker the RMS is, the less influence of the substrate is given. Furthermore, it could be mentioned that RMS on 125 µm Kapton® had a slower reaction front than the samples on 50 µm Kapton®, see Fig. 5. An explanation for this can be found in the substrate of the samples. Within the roughness measurements performed by LSM the surface of the 125 µm Kapton® was much rougher than the surface of the 50 µm Kapton®. This affects the growing of the RMS during the sputtering process massively. The higher the substrate roughness the higher the interface and surface roughness which increases the chance of nodular and keyhole defects [28] in the deposited layers. These defects, which were also seen in Fig. 4d), in combination with the higher interlayer roughness leads to a hindering of the atomic interdiffusion which finally occurs to slower reaction front velocities [32,33,34,35]. It can be seen that the substrate thickness and the roughness have an influence on the self-propagating reaction front. However, it could not be clearly shown which parameter has the greater influence. In order to verify this, further investigations must be carried out with different substrate thicknesses and the same surface roughness. Moreover, different surface roughnesses should be investigated with the same substrate thickness. Thus, a weighting of the parameters can be better assessed.
In the case of the slight tendencies according to the induced extrinsic stress, it should be noted that this is at this moment not clearly attributable to the different stress conditions. The reason is that it cannot be excluded that possible fluctuations during the measurements and inhomogeneities in the Kapton® substrate as well as in the RMS itself, which can be attributed to the sputtering process, have influenced the measurement results to that extent. These possible mentioned inhomogeneities can influence the diffusion process, which hinders the heat release during the exothermic reaction and leads to changes in the reaction front velocities [23, 28].
The investigations of the reaction front velocity show that both the substrate, due to different roughness, and the total layer thickness of the RMS, in case of reactions on substrates, exhibit a much higher influence on the reaction velocity than a possible influence of the extrinsically induced tensile stress.
In case of the commercial Nanofoils® the reactions were carried out in a freestanding condition, which implies that in this case there is no influence from a substrate. Since the commercial Nanofoils® showed a much more uniform behavior according to applied strain compared to the self-produced RMS, see Fig. 5(b), it can be concluded that fluctuations during the measurement play a rather minor role. Despite the fact that no tendencies toward higher or lower velocities are discernable in the Nanofoils®, which would suggest that there is no influence of the applied strain in correlation to the reaction front velocity, this cannot be applied to the self-prepared films, since a scaling effect from thinner RMS (1-5 µm) to thicker RMS (40-60 µm) in connection with their different behavior cannot be excluded.
Influence of extrinsic induced tensile stress on the reaction temperature
The adiabatic temperature of the reaction from Al + Ni to AlNi (B2 phase) is given by 1639 °C [31]. There is a large difference between the measured temperature, see Figs. 6 and 7, and the adiabatic temperature, which could be due to several reasons, such as the measurement equipment. The emission coefficient of the pyrometer was set to ε = 1, what corresponds to an ideal blackbody radiation emitter. However, this property does not apply to the Al/Ni RMS, which means that the measured temperature is always lower than the real value. Furthermore, the measuring wavelength of the pyrometer is given by 2.0-2.2 µm, which leads to an underestimating of the measured temperature [36]. In addition, due to the sputtering process the RMS of Al and Ni typically contains an intermixing area at the interfaces, which leads to a decrease in the maximum temperature of the system during the reaction [9, 31, 37]. Further, it is also possible that heat losses to the environment occur, which inhibits the reaction from achieving the adiabatic temperature [23, 38]. All these effects show the same tendency toward lower temperatures, which accounts for the large difference from the adiabatic temperature to the measured ones. Due to the very similar maximum temperatures it can be stated that an extrinsically induced mechanical stress has a nonsignificant influence on the temperature regime of the reaction in the local region of observation. However, it cannot be ruled out that the temperature distribution over the whole sample does not change with applied strain, as only local measurements were possible. For an extensive thermal characterization of the samples a thermographic camera with high resolution in the µs area would have to be used.
Influence of extrinsic induced tensile stress on the formed phases
The results, shown in Fig. 8, showed that the extrinsic induced stress does not lead to a variation of the formed phases. This means that the possible induced extrinsic tensile stress, without failing of the RMS by cracking, is too low to show some changes in the formed phases, like it is described by Cahn [27]. But it cannot be excluded that a minor amount of different phases has formed, as they are below the detection limit of our XRD measurements. For justification, it is necessary to perform further analyses with a higher resolution.
Conclusions
In this study, self-produced nanolayered Al/Ni multilayers by sputter deposition as well as commercial Nanofoils® were investigated with respect to their properties during and after high-temperature synthesis when extrinsic tensile stresses were induced.
The criteria were mainly directed to the velocity of the self-propagating reaction as well as the maximum temperature during and phases formed after the reaction. It could be shown that the induced extrinsic tensile stresses neither significantly affect the velocity of the propagation front nor the maximum temperatures reached. Moreover, XRD analysis showed no obvious differences in the formed phases after the reaction of the RMS. It should be noted that these results cannot be applied to other stress regimes, so that further investigations for, e.g., extrinsic compressive or direction dependent transient stress states are necessary to verify these statements. Furthermore, it must be pointed out that the extrinsic stress induced in these studies is too low to have a significant effect on the self-propagating high-temperature synthesis or rather the layers failed in the form of cracking before the stresses reach a value that could show a significant effect. In the future studies, more focused investigations have to be conducted to gain a deeper understanding of the impact of the observed phenomenon on the microstructure and the underlying diffusion mechanism. These investigations are crucial for elucidating the fundamental mechanisms governing the observed behavior and can pave the way for the development of novel and optimized RMS with superior properties.
In summary, these results represent important insights for the application of the Al/Ni RMS in terms of its stability or reliability related to propagation front velocity, maximum temperature and formed phases under induced extrinsic tensile stresses.
Data availability
The data that support the findings of this study are available from the corresponding author, S. Matthes, upon reasonable request.
References
Bridges D, Rouleau C, Gosser Z, Smith C, Zhang Z, Hong K, Hu A (2018) Self-powered fast brazing of Ti-6Al-4V using Ni/Al reactive multilayer films. Appl Sci 8(6):985
Ramos AS, Maj L, Morgiel J, Vieira MT (2017) Coating of tungsten Wire with Ni/Al multilayers for self-healing applications. Metals 7(12):574
Wang J, Besnoin E, Duckham A, Spey SJ, Reiss ME, Knio OM, Weihs TP (2004) Joining of stainless-steel specimens with nanostructured Al/Ni foils. J Appl Phys 95(1):248–256
Kim K (2019) Numerical investigation of the self-propagation of intermetallic reaction waves in nanoscale aluminum/nickel reactive multilayer foils. Korean J Met Mater 57(2):97–107
Yi P, Falk ML, Weihs TP (2018) Intermetallic formation at deeply supercooled Ni/Al multilayer interfaces: A molecular dynamics study. J Appl Phys 124(16):165303
Turlo V, Politano O, Baras F (2017) Microstructure evolution and self-propagating reactions in Ni-Al nanofoils: An atomic-scale description. J Alloys Compd 708:989–998
Jayaraman S, Mann AB, Weihs TP, Knio OM (1998) A numerical study of unsteady self-propagating reactions in multilayer foils. Symposium (International) on Combustion. 27(2):2459–2467. https://doi.org/10.1016/S0082-0784(98)80098-2
Sauni Camposano YH, Riegler SS, Jaekel K, Schmauch J, Pauly C, Schäfer C, Schaaf P (2021) Phase transformation and characterization of 3D reactive microstructures in nanoscale Al/Ni multilayers. Appl Sci 11(19):9304
Ramos AS, Simões S, Maj L, Morgiel J, Vieira MT (2020) Effect of deposition parameters on the reactivity of Al/Ni multilayer thin films. Coatings 10(8):721
Świątek Z, Gradys A, Maj Ł, Morgiel J, Marszałek K, Mania R, Szlezynger M (2016) XRD and TEM heating of large period Ni/Al multilayer coatings. Acta Physica Polonica A 130(4):880–883
Morgiel J, Szlezynger M, Pomorska M, Maj Ł, Marszałek K, Mania R (2015) In-situ TEM heating of Ni/Al multilayers. Int J Mater Res 106(7):703–710
Urrutia A, Tumminello S, Lamas DG, Sommadossi S (2015) X-Ray Characterization of Intermetallic Phases in Al/Ni Multilayer System. Procedia Mater Sci 8:1150–1159
Crone JC, Knap J, Chung PW, Rice BM (2011) Role of microstructure in initiation of Ni–Al reactive multilayers. Applied Physics Letters 98(14):141910
Trenkle JC, Koerner LJ, Tate MW, Walker N, Gruner SM, Weihs TP, Hufnagel TC (2010) Time-resolved X-ray microdiffraction studies of phase transformations during rapidly propagating reactions in Al/Ni and Zr/Ni multilayer foils. J Appl Phys 107(11):113511
Julien B, Dubreuil P, Josse C, Salvagnac L, Pelloquin S, Esteve A, Rossi C (2021) Effect of substrate–induced localized stress on the combustion properties of Al/CuO reactive multilayer films. Thin Solid Films 740:139000
Ni J, Li J, Jian J, He J, Chen H, Leng X, Liu X (2021) Recent studies on the fabrication of multilayer films by magnetron sputtering and their irradiation behaviors. Coatings 11(12):1468
Knepper R, Fritz G, Weihs TP (2008) Controlling the shape of Al/Ni multilayer foils using variations in stress. J Mater Res 23(7):2009–2016
Reiss ME, Esber CM, Van Heerden D, Gavens AJ, Williams ME, Weihs TP (1999) Self-propagating formation reactions in Nb/Si multilayers. Mater Sci Eng A 261(1–2):217–222
Aziz MJ (1998) Pressure and stress effects on diffusion in Si. Defect Diffusion Forum 153–155:1–10
Aziz MJ, Zhao Y, Gossmann HJ, Mitha S, Smith SP, Schiferl D (2006) Pressure and stress effects on the diffusion of B and Sb in Si and Si-Ge alloys. Phys Rev B 73(5):054101
Abadias G, Chason E, Keckes J, Sebastiani M, Thompson GB, Barthel E, Doll GL, Murray CE, Stoessel CH, Martinu L (2018) Review article: stress in thin films and coatings: current status, challenges, and prospects. J Vac Sci Technol, A: Vac, Surf Films. https://doi.org/10.1116/1.5011790
Adams DP (2015) Reactive multilayers fabricated by vapor deposition: a critical review. Thin Solid Films 576:98–128
Gavens AJ, Van Heerden D, Mann AB, Reiss ME, Weihs TP (2000) Effect of intermixing on self-propagating exothermic reactions in Al/Ni nanolaminate foils. J Appl Phys 87(3):1255–1263
Balluffi RW, Blakely BR (1975) Special aspects of diffusion in thin films. Thin Solid Films 25:363–392
Bourim EM, Kang IS, Kim HY (2021) Investigation of integrated reactive multilayer systems for bonding in microsystem technology. Micromachines 12(10):1272
Prikhodko SV, Ardell AJ (2003) Coarsening of in Ni–Al alloys aged under uniaxial compression: II. Diffusion under stress and retardation of coarsening kinetics. Acta Mater. 51:5013–5019
Cahn RW (1989) Phase equilibria under stress. Adv Mater 300–302. https://doi.org/10.1002/adma.19890010812
Panjan P, Drnovšek A, Gselman P, Čekada M, Panjan M (2020) Review of growth defects in thin films prepared by PVD techniques. Coatings 10(5):447
Wu H, Mayeshiba T, Morgan D (2016) High-throughput ab-initio dilute solute diffusion database. Scientific data 3(1):1–11
Edelstein AS, Everett RK, Richardson GY, Qadri SB, Altman EI, Foley JC, Perepezko JH (1994) Intermetallic phase formation during annealing of Al/Ni multilayers. J Appl Phys 76(12):7850–7859
Weihs TP (2014) Metallic films for electronic optical and magnetic applications. Elsevier, USA, pp 160–243
Liu B, Yu X, Jiang X, Qiao Y, You L, Wang Y, Ye F (2021) Effect of deposition substrates on surface topography, interface roughness and phase transformation of the Al/Ni multilayers. Appl Surf Sci 546:149098
Tien C-L, Lee P-W, Lin S-C, Lin H-Y (2021) Surface roughness and residual stress evolution in SiNx/ SiO2 multilayer coatings deposited by reactive pulsed magnetron sputtering. Opt Appl. 223–233. https://doi.org/10.37190/oa210206
Miyagaki S, Yamanashi H, Yamaguchi A, Nishiyama I (2004) Influence of glass substrate surface roughness on extreme ultraviolet reflectivity of Mo/Si multilayer. J Vac Sci Technol B 22(6):3063–3066
Trost M, Schröder S, Feigl T, Duparré A, Tünnermann A (2011) Influence of the substrate finish and thin film roughness on the optical performance of Mo/Si multilayers. Appl Optics 50(9):C148–C153
Woll K (2012) Festkörper-und selbstfortschreitende Reaktionen in Multilagen zur RuAl-Dünnschichtsynthese. PhD Dissertation, Universität des Saarlandes
Noro J, Ramos AS, Vieira MT (2008) Intermetallic phase formation in nanometric Ni/Al multilayer thin films. Intermetallics 16(9):1061–1065
Simões S, Viana F, Ramos AS, Vieira MT, Vieira MF (2009) Intermixing in Ni/Al multilayer thin films. Microscopy and Microanalysis 15(S3):75–76
Acknowledgements
This work is supported by the Deutsche Forschungsgemeinschaft (DFG grants Scha 632/29, Scha 632/30, BE 3198/7 and BA 6161/1). Many thanks to Joachim Döll from the Center of Micro- and Nanotechnology (ZMN), a DFG-funded core facility at TU Ilmenau (DFG Scha 632/27), for his professional help in the deposition of the samples.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical Approval
No experiments involving human participants and/or animals were carried out.
Additional information
Handling Editor: P. Nash.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matthes, S., Glaser, M., Vardo, E. et al. Influence of extrinsic induced tensile stress on the self-propagating high-temperature synthesis of nanosized Al/Ni multilayers. J Mater Sci 58, 10085–10095 (2023). https://doi.org/10.1007/s10853-023-08618-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08618-w