Abstract
Reactive aluminum–nickel multilayer system shows exothermic energetic materials which act as a heat source for packaging and bonding of microsystems. The main challenge is controlling the self-propagation reaction velocity and temperature generated by thermal management through different thermal conductive substrate materials. The current work investigates the heat distribution of Al/Ni multilayer foils from different thermal conductive substrates which act as heat sink materials during the self-propagating reaction. A two-dimensional numerical model was developed to study thermal conductive heat loss and substrate thermal properties on the self-propagating reaction in Al/Ni multilayer foils. The self-propagating reaction was introduced on the surface of the foils by an electrical spark. Here we investigate the minimum critical thickness of Al/Ni multilayer foils which shows the self-propagation reaction on different substrates and verified from the two-dimensional numerical model. The outcomes of this investigation will facilitate the integration of Al/Ni multilayer foils on different substrates as intrinsic heat sources for different applications of micro/nanodevices.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Self-propagating exothermic reaction is a well-known process, where chemically active systems react and produce heat and a solid product [1, 2]. The produced heat is up to hundreds of kJ per mole of atoms [3], and it is usually accompanied by a bright diffusion flame. Potential applications for self-propagating exothermic reactions are in the semiconductor industry [4], joining [5], etc. For joining different substrates, reactive nano multilayer uses as a heat source [6]. Normally, the reactive Al/Ni multilayer system certainly consists of hundreds of alternating layers of two or more different reactant layers combined as metal/oxide, metal/metal, or metal/metalloid [7, 8]. The exothermic reaction due to the interdiffusion of adjacent material layers acts as a thermal energy source for bonding [4]. In the consideration of local and instant heat sources for solder bonding, Al/Ni has impressive exothermic characteristics [9, 10]. So far, different modeling approaches have been studied in the literature. In the current study a two-dimensional (2D) numerical model with the help of COMSOL MULTIPHYSICS 5.6 of diffusion-limited reaction of Al/Ni multilayer foils is developed, using finite element methodology and compared with experimental results. As the developed model in the current study, investigates the effect of characteristics of reaction propagation in multilayer foils.
Experimental
Al/Ni multilayer fabrication method
To prepare Al/Ni multilayers, different types of substrates were used like Si(100) p-type doped; 1 µm wet oxidized SiO2 on top of p-type Si (SiO2/Si); 50 µm thick Kapton and quartz substrate. First, remove of external particles and impurities from substrate materials to prepare for the deposition of Al/Ni multilayers. DC magnetron sputtering (CS400 by von Ardenne) was used for deposition, where nickel, (99.99% purity, FHR) and aluminum (99.99% purity, FHR) 100 mm diameter targets were used. Alternating layers of Al and Ni of different total thicknesses from 1 to 5 µm of Al/Ni multilayer with 50 nm bilayer thickness (30 nm Al and 20 nm Ni layers thicknesses) were deposited at room temperature. 5 µm Al/Ni free-standing multilayer prepared by mechanical peel-off from the silicon substrate. An electrical spark was used with 16 V applied voltage. The electrical probe was placed at the edge of the prepared sample. For the numerical model, a 1011 W m−2 heat pulse for 1 µs was applied at the edge of the prepared sample, to make sure the initiate self-propagating reaction even though it has an insignificant thermal effect due to the igniter. The front velocity of the self/propagating reaction was recorded from a high-speed camera (FASTCAM SA-X2) with a 50,000 fps frame rate. To measure reaction temperature, a high-speed pyrometer (KLEIRER—Pyroscope 840 pyrometer) was used.
Modeling and simulation approach
A two-dimensional simulation model is applied to characterize the self-propagating diffusion reaction between Al and Ni multilayers. As shown in Fig. 1a multilayers of Al (30 nm) and Ni (20 nm) with bilayer (δ) thickness of 50 nm are alternatively present. W is the intermixed layer that formed between Al and Ni due to intermixing during deposition and in this model, it is 4 nm. The computational domain, thermal boundary conditions as radiative, convective heat losses, mass and heat transfer, diffusivity, etc. are used [11]. The developed model in [11] was used in the current study to evaluate the effect of different substrates on the characteristics of Al/Ni multilayers. For numerical simulation, the thickness of Si is set to 50 µm instead of 525 µm like an experiment to reduce the calculation cost. It is also reliable because for Si, the thickness of the numerical heat-affected zone is calculated and it is 40 µm only [11]. Heat-affected zone is the area where the heat will be spread due to the exothermic reaction of Al/Ni multilayers and beyond that the temperature gradients are nearly zero. The properties of the substrate for simulation were taken from the room temperature properties. This is a logical assumption because multilayer foil reactions are normally very fast accompanying partial or complete delamination of the foils from the substrates.
Result and discussion
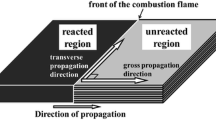
Figure 1b shows the reaction front of the 5 µm thick Al/Ni multilayer as a free-standing foil. As we can see from Fig. 1b, the self-propagating exothermic reaction front emits radiation (brightness) which does not rapidly decrease behind the reaction front. Once the multilayer is ignited locally, the interdiffusion and eventually intermixing of Al and Ni emits large amounts of energy, which can initiate a self-propagating reaction wave [12]. The reaction front propagation is governed by a combination of crystallization and diffusion of the interlayers. Furthermore, it is demonstrated that the heat of crystallization of Al and Ni to B2-AlNi individually is pretty enough for a self-propagating reaction to happen as shown in Fig. 1b. At the reaction front in Al/Ni multilayers, the Al layer melts first, meantime the intermixed (w) interlayer begins to crystallize. Just after the reaction front, volume diffusion occurs from the w interlayer into the melted Al layer. Simultaneously, atoms from the w interlayer begin to diffuse into the grain boundary of the Ni. Afterward, behind the reaction front, the Ni layer starts to diffuse into the crystallized layer and eventually, all the interlayers of Al and Ni crystallize into B2-AlNi [13].
As shown in Fig. 1c, Al/Ni multilayers were deposited on the different substrates and performed an ignition test. It was observed that the behavior of exothermic reaction was varying from substrate to substrate, which shows the reaction front can be controlled by thermal management to manage the heat loss through the substrate [12]. Reactive foil on a specific substrate, where the substrate act as a heat sink by taking the heat away from the reaction front. These extra conductive losses impede the propagation of the reaction. Additionally, if conductive losses are too much, it can result in a highly nonlinear heat release from the reaction site. Consequently, quenching of the heat wave, therefore rendering the heat source ineffective. As shown in Fig. 1c, Al/Ni multilayers were grown on different substrates such as kapton, quartz, 1 µm SiO2 on the top of Si substrate, and silicon substrate which have different thermal conductive values of 0.12 W mK−1, 1.37 W mK−1, 1.38 W mK−1 and 148 W mK−1 respectively. From the ignition test, it was observed that the critical thickness of the Al/Ni multilayers which shows the self-propagation reaction changed for the different substrates. Figure 1d showed the variation between the critical thickness of the Al/Ni and the thermal property of different substrates. It is demonstrated that for very high thermal conductive (λ) substrates like silicon and glass, the critical thickness of the Al/Ni multilayers is 10 µm and 4 µm, respectively.
However, critical thickness is decreased in the case of lower λ substrate like kapton which is 1 µm enough to show a self-propagation reaction. The self-propagation reaction on the kapton (very low λ) substrate is equivalent to previously reported research [14, 15]. The less λ substrates demonstrated the absence of conductive heat losses. The varriation of critical thickness with different substrates is summarised in Fig. 1d and it shows a good agreement between observed experimental results with simulations.
It can be concluded that for the reduction of the total layer thickness needed to achieve a self-propagating high-temperature reaction either (1) the stored energy has to be increased, (2) the necessary reaction temperature has to be reduced or (3) the thermal conductivity of the substrate has to be reduced. The first two conditions can be achieved by appropriate changes in the material combinations in the bilayer system. The third requirement can be fulfilled by tuning the substrate thermal conductivity through an appropriate layer stack or other types of processing.
Figure 2a and d show the schematic of the 1 µm and 5 µm Al/Ni multilayers on a 1 µm SiO2 insulating layer on Si substrate, respectively. Figure 2c, showed the calculated temperature distribution of the multilayers diffusion reaction on 50 µm thick SiO2 on top of 50 µm thick Si is illustrated. With this, the effect of increasing the thickness of thermal insulation (SiO2 layer) is numerically calculated. For the case where a 1 µm thick multilayer foil is placed on top of a 50 µm thick insulating layer on a Si substrate, increasing the thickness of the insulating layer is not sufficient to prevent quenching of the reaction. Figure 2c is representing the quenched reaction where the temperature at the beginning (t = 2 µs) near the left boundary is high because of the ignition initiation condition. As explained, the ignition initiator will be deactivated after 1 µs from the beginning of calculations. So it is apparent that the initial rise in temperature is due to the igniter effect. The maximum temperature is decaying with time and with the distance from the ignition point (x = 0 µm). So, no self-propagating reaction occurs. The reason for this behavior is that the conductive heat loss is so high that it could not be overcompensated by the heat released from the exothermic chemical reaction and the stored heat in the 1 µm thick multilayer foil as demonstrated in Fig. 2b. When the volume of multilayer foil, i.e., the total thickness of the multilayer foil is increased, it is expected that a cross-over between the heat loss and heat release of the exothermic reaction can be reached. Beyond the cross-over point, the time and space-dependent temperature distributions will change. The simulated temperature distributions were obtained for a 5 µm thick multilayer foil placed on top of a 1 µm thick insulating SiO2 on top of a 50 µm thick Si substrate. As it is evident in Fig. 2f, the reaction front is moving along the x-axis evidenced by a non-decaying temperature profile with a box-type temperature distribution and a temperature maximum at the end, i.e., at the largest distance from the initiation point. The maximum originates from the ongoing self-sustained chemical reaction acting as the primary heat source moving with a certain velocity, i.e., the propagation velocity of the self-propagating reaction. The dramatic drop in temperature is the effect of passing the reaction wave. The velocity of the reaction front is calculated by dividing the difference of the reaction front position along the x-axis by the time difference. The obtained reaction front velocity is equal to 7.61 m s−1 and the reaction front showed in Fig. 2e, which is slower than 9.44 m s−1of free-standing samples. This difference shows the thermal effect of the substrate on the characteristics of the reaction which is well predicted also by the numerical model adopted in the current work.
a Schematic of 1 µm multilayer foils on SiO2/Si substrates; b Indication of no self-propagation achieved from (a); c Temperature vs x-axis plot for different times after the reaction initiation for a 1 µm and thick multilayer foil on top of 50 µm thick SiO2 on a 50 µm thick Si substrate; d Schematic of 5 µm multilayer foils on SiO2/Si substrates; e Reaction front at the different time frame of 5 µm Al/Ni foil on SiO2/Si substrate. f For a 5 µm thick multilayer foil on top of 1 µm thick SiO2 layer on 50 µm thick Si substrate. The x-axis is placed at the tMl/2 of the multilayer. The time steps t are in µs
The numerical heat-affected zone has also been shown in Fig. 3, which shows that heat dissipation is absent in the substrate as Kapton. This means that the multilayer foils with very low λ substrates react and self-propagate as in “free” standing conditions. However, SiO2/Si substrate, heat dissipated into the substrate as the reaction front moves forward. Therefore, the ignition front allows the front to introduce into the “quenching zone” [14], to ensure that the heat sink enables the stable reaction front with no ignition artifacts can alter the front velocity and temperature observed during propagation. Therefore it is indicated that the addition of a heat sink can simply alter the delicate energy balance for a self-propagating reaction. Indeed, the energy generated by the intermetallic exothermic reaction is gradually absorbed by the substrate of decreasing thickness of Al/Ni multilayers, until a point where the losses term dominates the energy releaser term. Consequently, it leads to quenching. A vital consequence of these results is that, despite the substrate heat sinks substantially affecting the quality of propagation reaction, a stable propagation front is still possible to achieve and the reaction propagates steadily as “partially quenched” conditions [14]. Additionally, the surface roughness of the substrate also plays a major role in affecting the interface roughness, surface topography, and phase transformation. The substrate roughness can considerably increase the interface roughness, and this effect continues for the later layers. Substrate roughness produces the gradient profile among interlayers, which delays the crystallization of the interlayers. Additionally, this enables for extra volume diffusion at the initial stages of the reaction and reduces the crystallized interlayer height, after it has formed. Consequently, the front propagation velocity for as-deposited Al/Ni system along with a gradient profile is slower than for the Al/Ni with the homogeneous profile. The intensely roughened surface of the substrate causes much rougher interlayers, lower surface flatness, and thus low compactness of the deposited multilayers [6]. Therefore, the pre-formed intermixed (w) layers would impede the diffusion of Ni and eventually slower the AlNi formation. In consequence, substrates could also impact the w region at interfaces. Smaller λ coefficient of the substrate like Kapton gives rise to sharper and clearer interfaces which further degrades the interface roughness. The roughness of the interface could be affected by the factors confining the mobility of the deposited atoms, for instance, surface diffusion [16]. A larger λ of the substrate is able to remove the effect of the kinetic energy of the incoming atoms during deposition, resulting in lower diffusivity of incoming atoms. From this perspective, it is contemplated that the deposition substrates are able to use for tailoring microstructure, atomic diffusion, and phase transformation in Al/Ni multilayers. The λ coefficient of the substrates also needs to be considered. An additional aspect that should be prominent is the delamination of the reactive multilayers from the substrates after the reaction. Normally, large internal stresses while the deposition could govern the delamination of the reactive multilayers from the substrate [17, 18]. Therefore, in a nutshell, stress, substrate roughness and thermal expansion coefficient, also affect interface binding between the substrates and multilayers.
To check with numerical simulation, different thicknesses of multilayer foils are placed on top of the substrate. In Fig. 4, demonstrated that the reaction front velocity and the reaction temperature are plotted dependent on the λ of the substrate for 1 and 5 µm total thicknesses of Al/Ni multilayers. It is clear from Fig. 4 that increasing the total thickness of multilayer foil sustains the self-propagating reaction on substrates with larger λ. So, the reaction zone as it is illustrated with green boxes will be extended when the total multilayer foil thickness is increased.
Numerical calculations for certain λ values show irregular behavior, this range is illustrated in the blue boxes and it is called the transient zone. Although in the reaction zone with increasing thermal conductivity, the reaction front velocity and the reaction temperature is decreasing (this is due to larger heat dissipation through the substrate), in the transient zone there is no specific observed trend for velocity and temperature. The behavior in the transient zone might be due to calculation errors. For the 1 µm multilayer foil system (Fig. 4) the self-propagating reaction can be achieved for the substrate with thermal conductivity of 0.05 W m−1 K−1. On the other hand, in the system, which 5 µm foil on the substrate, the self-propagating reaction can be achieved up to thermal conductivities of 1.9 W m−1 K−1. This is due to larger total energy storage for thicker foils which can be released and resist heat dissipation through the substrate. This means that the temperature of the foils is high enough while the reaction not causing quenching of the reaction.
Conclusion
The numerical model was developed and updated in a way that the calculation domain contains the multilayer foils on a substrate. The simulation results were compared with the existing experimental data to verify the numerical model. Effective properties of substrate material on the characteristics of the reaction in the foil were investigated. It was found that increasing the thermal conductivity of the substrate material lower the temperature of the reaction and can eventually, causes the reaction to quench. The simulation results showed that for the multilayer foils fabricated on the kapton, the reaction occur and sustained the self-propagating mode for the foils with minimum total thicknesses of 1 µm. But for the foils placed on the top of SiO2/Si substrates, the minimum total thickness was 5 µm to show a self-propagating reaction but with less than 5 µm total thickness the reaction was quenched immediately after the beginning initiation. In conclusion, this result indicates that substrates may provide advantages to modify the performance of the multilayers.
Data availability
Data will be available upon the request.
References
M.A. Hobosyan, K.S. Martirosyan, IEEE Nanotechnol. Mag. 14, 30 (2019)
G.M. Fritz, S.J. Spey, M.D. Grapes, T.P. Weihs, J. Appl. Phys. 113, 014901 (2013)
S. Bhattacharya, A.K. Agarwal, T. Rajagopalan, V.K. Patel, Nano-Energetic Materials (Springer, Cham, 2019). https://doi.org/10.1007/978-981-13-3269-2_13
J. Braeuer, J. Besser, M. Wiemer, T. Gessner, Sens. Actuator A 188, 212 (2012)
J. Braeuer, J.Besser, M.Wiemer, T. Gessner, In Proceedings of the 2012 4th Electronic System-Integration Technology Conference, Amsterdam, The Netherlands, 17, 1–4 (2012)
E.-M. Bourim, I.-S. Kang, H.Y. Kim, Micromachines 12, 1272 (2021)
D.J. Fisher, Materials Research Forum LLC: Millersville, PA, USA, 2019; ISBN 978-1-64490-009-3
T.S. Dyer, Z.A. Munir, V. Ruth, Scr. Metall. Mater. 30, 1281 (1994)
T. Namazu, H. Takemoto, H. Fujita, Y. Nagai, and S. Inoue, Proc. 19th IEEE Int. Conf. Microelectromech. Syst. (MEMS), 286 (2006)
X. Qiu, J. Wang, Sens. Actuators A 141, 476 (2008)
M. Baloochi, D. Shekhawat, S.S. Riegler, S. Matthes, M. Glaser, P. Schaaf, J.P. Bergmann, I. Gallino, J. Pezoldt, Materials 14, 7815 (2021)
F. Schwarza, R. Spolenak, J. Appl. Phys. 131, 075107 (2022)
A. Rogachev, S. Vadchenko, F. Baras, O. Politano, S. Rouvimov, N. Sachkova, A. Mukasyan, Acta Mater. 66, 86 (2014)
S. Danzi, M. Menetrey, J. Wohlwend, R. Spolenak, ACS Appl. Mater. Interfaces 13, 42479 (2019)
D.P. Adams, Thin Solid Films 576, 98 (2015)
P. Politi, G. Grenet, A. Marty, A. Ponchet, J. Villain, Phys. Rep. 324, 271 (2000)
R. Knepper, G.M. Fritz, T.P. Weihs, J. Mater. Res. 23, 2009 (2008)
W.D. Westwood, Sputter deposition (AVS, New York, 2003)
Acknowledgments
The authors express special thanks of gratitude to Marcus Glaser and Sebastian Matthes TU Ilmenau, for their help and support for high-speed camera measurement and high-speed pyrometer. Support by the Center of Micro and Nanotechnologies (ZMN), a DFG-funded core facility of TU Ilmenau, is also gratefully acknowledged. The authors acknowledge the financial support of this research by the German Science Foundation under contract numbers PE 624/16-1, SCH A632/29-1, SCHA 632/30-1, BE 3198/7-1, and JA 1023/14-1. We acknowledge support for the publication costs by the Open Access Publication Fund of the Technische Universität Ilmenau.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shekhawat, D., Baloochi, M., Sudhahar, D. et al. Influence of environment on self-propagating reactions in Al/Ni multilayer foils. MRS Advances 8, 477–483 (2023). https://doi.org/10.1557/s43580-023-00574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00574-6