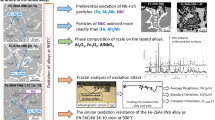
Abstract
Complex concentrated alloys (CCAs) are attracting considerable interest due to their potential applications under extreme conditions. This study focuses on two complex concentrated alloys, the FeAlCrV and the FeAlCrMo alloys, which already exhibited exceptional mechanical properties at high temperatures. In this regard, room temperature corrosion resistance and high-temperature oxidation were studied to investigate their potential applicability in harsh environments. It is shown that the corrosion resistance of both CCAs is much higher than that of AISI 304 and P91 steels in 0.5 M H2SO4 solution, while in 3.5% NaCl solution was comparable. On the contrary, high-temperature oxidation of CCAs was unsatisfactory, especially exceeding 700 °C. The intensive analysis of the formed oxide scales revealed that the protective oxide layer is not being formed at temperatures above 700 °C, primarily because of the occurrence of vanadium corrosion (FeAlCrV) and evaporation of Mo oxides (FeAlCrMo). The results of this study unambiguously showed the importance of studying oxidation properties at high temperatures parallel with the mechanical properties for the development of CCAs for cutting-edge technical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The massive development in cutting-edge technical applications, including gas turbines, jet engines and power plants, requires intensive development of materials resistant to extreme conditions with adequate mechanical and oxidation resistance [1]. Recently, complex concentrated alloys (CCAs), consisting of multiple elements in equimolar or near-equimolar ratios [2], are considered a potential candidate for such demanding applications. Specifically, some CCAs are believed to replace Ni-based high-temperature alloys [3, 4]; several studies have identified CCAs with excellent corrosion resistance surpassing stainless steels [5,6,7] and CCAs with improved mechanical properties in comparison with conventional alloys [8, 9]. However, complex, thorough characteristics of CCAs are still missing, for example studies dealing both with oxidation and mechanical properties at elevated temperatures [4]. This problem can be directly seen in the CrMnFeCoNi alloy (also referred to as Cantor alloy). Despite outstanding mechanical properties such as high ductility, work hardening rate and ultimate tensile strength [10, 11], alloy shows poor oxidation resistance due to the high oxidation rate caused by porous oxide layers [12].
In this study, we focused on further characterization, namely oxidation behaviour, of two recently developed CCAs, FeAlCrV and FeAlCrMo alloys. These alloys have exhibited excellent mechanical properties, even at high temperatures. Maximum compressive stress at ambient temperature exceeds 1300 MPa for the FeAlCrV alloy and 1480 MPa for the FeAlCrMo alloy. When exposed to a temperature of 800 °C, the FeAlCrV and FeAlCrMo alloys show maximum compressive stress of 1210 MPa and 1030 MPa, respectively [13]. Because of these promising properties, we aim to study corrosion at room temperature and high-temperature oxidation to investigate the applicability of these alloys.
Constituting elements Mo and V fall into the class of refractory metals (RMs), characterized by high melting points and high elastic modulus even at high temperatures. Nevertheless, a fundamental issue of RMs is their oxidation resistance at elevated temperatures, as they tend to form oxides with low melting points and/or volatile oxides [14]. Namely oxide MoO3 tends to evaporate above 650 °C [15]. V2O5 has a melting point of 675 °C and allows the diffusion of oxygen [16]. Above that, V2O5 can react with chromia and alumina scale and thus degrade protective ability. This phenomenon is known as vanadium corrosion [17]. Regarding the concept of complex concentrated alloys, one may ask whether similar effects will be observed in the concurrent presence of V or Mo and other elements in high concentrations. Therefore, this study aims not only to determine the oxidation behaviour of the FeAlCrV and FeAlCrMo alloys but also to validate whether their oxidation properties may be predicted based on the knowledge regarding the oxidation of conventional alloys.
Experimental
Material
Ingots of the FeAlCrV and FeAlCrMo alloys were prepared by arc melting in an argon atmosphere. Each ingot was flipped over and re-melted ten times to obtain a homogenous composition. The purity of all constituent elements exceeds 99.8 at. %. In the cases of Fe and V, 0.1 at. % of C was detected. The composition was determined by a chemical wet procedure in an atom absorption spectrometer; the average values are listed in Table 1. Conventional AISI 304 steel (with a nominal composition of 69Fe-19Cr-8Ni-2Mn-1.7Si-0.3C [at. %]) and P91 steel (with a nominal composition of 80Fe-9Cr-0.8Mo-0.2Mn [at. %]) were chosen as the reference materials.
Microstructure
The microstructure and structure of the oxide layers of all studied samples were investigated by scanning electron microscope (SEM) Zeiss Auriga Compact equipped with EDAX energy-dispersive X-ray spectroscope (EDS). The samples for investigation of the oxide layer cross-sections were embedded in the conductive epoxy resin. All samples were mechanically polished down to 50-nm alumina suspension before the SEM analysis.
Corrosion resistance
The corrosion resistance of the investigated alloys was measured by potentiodynamic polarization tests using potentiostat Autolab PGSTAT128N and a three-electrode setup. A saturated calomel electrode (SCE) was used as a reference electrode for all measurements. Two corrosion media were used − 3.5% NaCl solution and 0.5 M H2SO4 solution, both prepared from deionized water. All tests were performed at room temperature after 30 min of stabilization. The tests were carried out in the potential range of − 0.25 V–1.3 V with respect to the open circuit potential (OCP), with a scan rate of 2 mV/s. During the test, the solution was stirred by a magnetic stirrer at 300 rpm. At least three tests were performed for each alloy/solution, and the samples were polished with emery paper (P1200, 15 µm) before each test.
High-temperature oxidation
For the oxidation tests, the FeAlCrV and FeAlCrMo samples of dimension 5 × 5 × 0.8 mm were polished up to 3 μm. As a control material, a disc-shaped sample of steel AISI 304 (radius 12 mm, thickness 1 mm) and a block-shaped sample of P91 steel (10 × 10 ×1 mm) were used. The samples were exposed to laboratory air at four different temperatures: 600 °C, 700 °C, 750 °C and 800 °C. During the test, specific mass gain (with an interval of 25 or 50 h) was measured.
Results and discussion
Microstructure
The original microstructure of all investigated alloys is shown in Fig. 1. In the FeAlCrV and FeAlCrMo alloys, grains of the size of several hundred microns were observed. The FeAlCrMo alloy was prepared in a single-phase solid solution with a dendritic substructure. The apparent dendrites are composed of the predominant occurrence of molybdenum. Note that small black particles with a low volume fraction (< 1%) were identified in the previous study as undissolved aluminium [13]. In the FeAlCrV sample, secondary phase V2C (denoted by the arrow in Fig. 2b) and VxCr1-x (0.8 < x < 1) occurred [13]. Particles of VxCr1-x are too small to be clearly distinguished in the SEM image and were previously identified by transmission electron microscopy; for detail, see Ref. [13]. Both steel samples, AISI 304 and P91, contain highly deformed grains of the size of several hundred microns.
Corrosion resistance
Typical polarization curves obtained for the studied samples in 3.5% NaCl solution and 0.5 M H2SO4 are displayed in Figs. 3 and 4, respectively. The passive region is delimited by points on polarization curves. The evaluated corrosion parameters are listed in Tables 2 and 3, namely corrosion current density and corrosion potential (icor and Ecor), critical current density for passivation and passivation potential (ipas and Epas) and transpassive current density and potential (itrans and Etrans).
In the 3.5% NaCl solution, the FeAlCrV and FeAlCrMo alloys exhibit almost the same corrosion current density icor as AISI 304. The corrosion potential of CCAs is less noble than that of the AISI 304 steel. On the contrary, steel P91 exhibits two orders of magnitude higher icor and is the least noble. In contrast with steels, CCAs are able to form a passive layer. In addition, the layer is stable at a potential range of about 0.4 V, and the critical current density for the formation of the layer is in the order of μA.
In the 0.5 M H2SO4 solution, the corrosion current density icor of CCAs is approximately 100 nA cm−2, roughly a hundred times smaller than that for the AISI 304 steel and a thousand times smaller than that for the P91 steel. The critical current density for passivation is also significantly lower for CCAs. On the other hand, both steel samples exhibit a significantly wider passive region in the polarization curve (in the case of the P91 steel, it is eight times wider than that of the FeAlCrV alloy) with a very low current density in the order of 10–4 A/cm2, similar to CCAs.
Discussion of the corrosion resistance
Potentiodynamic tests showed that the FeAlCrV and FeAlCrMo alloys' corrosion resistance in 3.5% NaCl solution is comparable with the AISI 304 steel in terms of the corrosion current density. However, unlike steels, they do not show a passive region in the potentiodynamic characteristic. In 0.5 M H2SO4 solution, according to corrosion and critical current densities, the investigated CCAs surpass both types of steels. The excellent corrosion resistance of CCAs is primarily due to their composition. Both contain about 25 at. % of Cr, while the AISI 304 steel contains 19 at. % of Cr and the P91 steel only 9 at. % of Cr. It is known that Cr is an essential alloying element in stainless steels for corrosion protection [18]. Cr's beneficial effect, which leads to satisfying corrosion resistance, was also already reported for various CCAs [19][19]. The effect of Al addition to CCAs is, however, still uncertain. It improves the corrosion resistance of CoCrFeNi in H2SO4 solution [5] but, on the contrary, reduces the corrosion resistance of CrFe1.5MnNi0.5 in both NaCl and H2SO4 solutions [21]. Mo addition to Fe–Cr alloys was proven to decrease critical current density as well as the passive current density in the H2SO4 solution [18]. On the other hand, some CCAs exhibit decreasing corrosion resistance with an increasing amount of Mo [22]. Nevertheless, in the case of TiZr0.5NbCr0.5, the addition of Mo and/or V leads to excellent corrosion resistance in the H2SO4 solution [7].
Despite various and contradictory effects of alloying elements, both FeAlCrV and FeAlCrMo exhibit similarly excellent corrosion behaviour in terms of corrosion, critical and passive current density and ability to passivate. Thus, the individual effect of V or Mo addition on the corrosion resistance of the FeAlCr alloys seems to be only minor. Regarding the different and non-homogeneous microstructures, it is noteworthy that surfaces of CCAs after corrosion (observed in SEM) did not exhibit any features typical of pitting corrosion, which agrees with the shape of the passive region in the polarization curves. Concerning excellent mechanical properties at room temperature (reported in [13]) and outstanding corrosion resistance in 0.5 M H2SO4 solution, FeAlCrV and FeAlCrMo alloys may be suitable for specific technical applications in harsh environments.
High-temperature oxidation kinetics
In Figs. 4 and 5, the mass gain is displayed as a function of time during the isothermal oxidation of studied alloys in air. Figure 4 represents the temperature effect on the oxidation kinetics of individual alloys, while Fig. 5 shows the comparison of alloys' oxidation at particular temperatures.
The results show that the mass gain was negligible for all the specimens during exposure to air at 600 °C. In the case of the FeAlCrMo alloy, the mass gain at 700 °C was higher but still very small. Therefore, the type of oxidation kinetics cannot be determined from the experimental data. Further increase in the temperature to 750 °C resulted in the kinetics development, which seems to follow a logarithmic rate law, see Fig. 4. Finally, the oxidation at 800 °C resulted in a non-typical oxidation curve. After 75 h of exposure, a relative mass loss was observed, which continued with the increase in the exposure time.
The FeAlCrV alloy generally exhibited higher mass gains than the FeAlCrMo alloy. During exposure to air at 700 °C, the oxidation kinetics followed a logarithmic rate law, whereas, during exposure to air at 750 °C and 800 °C, the kinetics became linear, as shown in Fig. 4.
In the case of AISI 304 steel, mass gains are negligible at all temperatures. On the other hand, P91 steel exhibits about ten times higher mass gains than the AISI 304 steel. However, it is apparent from Fig. 5 that mass gains for CCAs are significantly higher than those for both steel samples. This difference becomes more distinct with increasing temperature—the mass gain of the FeAlCrV alloy is a hundred times and four hundred times higher than that for AISI 304 after 100-h exposure to air at 750 °C and 800 °C, respectively.
Microstructural characterization of oxide scales
Alloys after exposure to air at elevated temperatures were further investigated—the morphology of the surface and the element distribution in oxide layers were studied by electron microscopy.
FeAlCrV
Figure 6 displays the cross-section BSE (backscattered electrons) images and an EDS mapping of the FeAlCrV alloy after 200 h of oxidation at 750 °C in air. The metallic substrate is covered by a multilayered oxide scale. The uppermost uneven layer (I) consists of Fe oxides. The second layer (II) is enriched in V and Fe, while the third layer consists of Al, Cr and V oxides. The fourth inhomogeneous layer (IV) contains dark "veins" enriched in Al, and the brighter matrix is enriched in V and Cr. The scale spallation was observed, which is demonstrated in the surface BSE image (Fig. 6b)—note that layer (I) is indistinguishable from layer (II).
During 100 h of exposure of the FeAlCrV alloy to air at 800 °C, a brittle oxide layer was formed, which subsequently partially spalled off, as shown in Fig. 7. EDS analysis confirms similar element distribution in multilayer oxide scale as that after exposure at 750 °C: the uppermost thin Fe layer followed by Fe and V oxides. The middle layer is depleted in V and consists of mixed oxides. The bottom layer is adhesive to the metal substrate and is depleted in Fe. Al and Cr oxides seem to be complementary with V oxide, as shown in Fig. 7.
It is apparent from Figs. 6 and 7 that all alloying elements participated in the oxidation process and that the structure of the formed oxide layer is complex. The EDX point analysis reveals the presence of various binary and ternary oxides that were not further identified. Nevertheless, observed cracks and voids indicate that the layer is composed of non-protective oxides, which is consistent with the mass-gain data (Figs. 4 and 5). It may be assumed that the V2O5 oxide was formed and subsequently reacted with other Al, Cr and Fe oxides, which resulted in the complex layer, enabling further diffusion of oxygen. Such a detrimental effect of vanadium was also observed in another CCA—the AlTiVCr alloy, which was unable to form a protective layer despite the high content of Al and Cr [23].
FeAlCrMo
After 200 h of exposure of the FeAlCrMo alloy to air at 750 °C, a two-layer oxide scale was observed, see Fig. 8. The uppermost layer (I) consists of Fe oxides. The second layer (II), about six times thicker, exhibits a fine structure: Thin dark sublayers are enriched in Al and Cr, whereas the light matrix is formed from Mo oxides. After exposure to air at 800 °C (Fig. 9), we observed significant changes in the oxide scale: The uppermost layer (I) thickness increased to the same extent as that of the second layer (II), and complete depletion of Mo in the layer was detected. The second layer has the same element distribution as for the exposure at 750 °C (Fig. 8).
As reported above, a relative mass loss was observed during exposure of the FeAlCrMo alloy to air at 800 °C, and Mo depletion in the uppermost layer was detected. That could indicate the evaporation of MoO3, which tends to evaporate at 650 °C [15]. To validate this hypothesis, we performed an additional test. The FeAlCrMo sample was placed into a glass tube and put into the chamber furnace through the hole in the furnace door. One part of the tube with the sample was inside the furnace, and the other one was stuck out of the door and cooled by the laboratory air. The furnace was heated up to 800 °C. After 100-h exposure, we removed bright crystals that developed inside the cooled part of the tube. As demonstrated in Fig. 10, these crystals consisted of Mo oxides which proved our assumption about evaporation.
Evaporation of MoO3 was also detected in another CCA with a similar composition—the MoCrTiAl alloy [24]. A similar oxide layer was also reported for the MoWAlCrTi alloy [25], where a high amount (overall 40 at. %) of Cr and Al did not lead to the formation of a continuous passive layer. Depletion of Mo in the uppermost layer was detected as well, but possible evaporation of MoO3 (falsely lowering the mass gain) has not been discussed.
Discussion of the high-temperature oxidation
The experimental data on kinetics reveal high mass gains and linear rate law indicating the formation of a non-protective oxide scale [18]. Microstructural analysis of oxide scales displays nonadherent brittle layers with numerous cracks. Thus, it is evident that examined CCAs suffer from poor resistance to oxidation at temperatures above 750 °C. Our study reveals that despite the high amount (altogether 50 at. %) of passivating elements Al and Cr, the presence of Mo and V impairs the beneficial effect of these elements. This result concurs well with previously studied CCAs AlTiVCr [23] and MoWAlCrTi [25]. Each of these alloys contains overall at least 40 at. % of Al and Cr but the protective layer is not established.
One may note that whereas conventional Fe–Cr-Al alloys (with Fe as a principal element and minor additions of Cr and Al) generally possess excellent oxidation resistance up to 1200 °C [26], CCAs containing Fe, Al and Cr may exhibit various degrees of protection against oxidation. On the one hand, adding a small amount of Ti (1 at. %) to AlCoCrFeNi results in even lower mass gain than that of conventional Fe–Cr-Al alloys [27]. On the other hand, a similar alloy with higher amount of Ti, Al0.5CoCrFeNi1.6Ti0.7, is not able to form a protective oxide layer during oxidation at 900 °C [28]. An excellent oxidation resistance can be reached by substitution of Ti with Cu, such as in the equiatomic AlCoCrFeNiCu alloy [29]. On the contrary, replacing Ti with V leads to an insufficient resistance of the Al0.25CoCrFeNiV alloy [28], which is in agreement with our findings. Taken together, it may be suggested that an oxidation resistance of CCAs containing Fe, Cr and Al is highly sensitive to the alloy composition.
It is clear that engineering application of the FeAlCrMo and FeAlCrV alloys at high temperatures is unattainable without alloy improvement. Good oxidation resistance is for CCAs, as for conventional alloys, determined by the formation of a continuous protective oxide scale such as chromia or alumina [23]. That may be achieved in various ways based on alloying or surface coating and surface modification, alternatively [1]; however, discussion of surface methods is beyond the scope of this work. Considering only the alloy composition, a sufficient amount of Al and Cr is needed. This condition is probably already satisfied in both investigated CCAs. Therefore, further research may be focused on microalloying—it was shown that the addition of rare earth elements such as Y and Ce impacts oxidation resistance by decreasing the growth rate of the alumina scale [30] and enhancing the adherence of the chromia and/or alumina scale [1]. A similar effect was also observed in alloys microalloyed with Si—the Si addition promotes Cr2O3 or Al2O3 formation and concurrently prevents V2O5 formation [31] [32]. On the other hand, Si can unfavourably affect the stability of the microstructure and mechanical properties, namely ductility. Nevertheless, it is conceivable that cautious microalloying may improve oxidation resistance and simultaneously preserve excellent mechanical strength at high temperatures.
Conclusion
In this paper, corrosion and high-temperature oxidation behaviour of complex concentrated alloys FeAlCrMo and FeAlCrV were investigated. The main findings can be summarized as follows:
-
Studied alloys show sufficient corrosion resistance in NaCl solution and remarkably high (in comparison with steels AISI 304 and P91) corrosion resistance in H2SO4 solution at room temperature. Passivation of CCAs occurs at low current densities in both solutions. On the other hand, the passive region in the H2SO4 solution is significantly shorter than that of steels AISI 304 and P91.
-
The high-temperature oxidation resistance of both investigated CCAs is unsatisfactory, despite the high content of Al and Cr. Both alloys exhibit distinctively higher mass gains than both steel samples. It was found that the protective oxide layer on CCAs is not being formed at temperatures exceeding 700 °C. A uniform layer of Al2O3 nor Cr2O3 was not identified in the cross-section analysis, and the upper layers consist of unfavourable Fe oxides. In the FeAlCrV alloy, vanadium corrosion probably occurred, resulting in a porous, non-protective layer composed of various oxides. In the FeAlCrMo alloy, evaporation of Mo oxides was observed. These results support the assumption that the presence of Mo and V in CCAs impairs the effect of Al and Cr.
-
The findings of this study show the necessity for further investigations on the mechanism of high-temperature oxidation of complex concentrated alloys in this class of CCAs.
Data and code availability
The data that support the findings of this study are available from the corresponding author, E.J., upon reasonable request.
References
Khanna AS (2002) Introduction to high temperature oxidation and corrosion. ASM International, Materials Park, OH
Miracle DB, Senkov ON (2017) A critical review of high entropy alloys and related concepts. Acta Mater 122:448–511. https://doi.org/10.1016/j.actamat.2016.08.081
Senkov ON, Miracle DB, Chaput KJ, Couzinie J-P (2018) Development and exploration of refractory high entropy alloys—a review. J Mater Res 33:3092–3128. https://doi.org/10.1557/jmr.2018.153
Chen J, Zhou X, Wang W et al (2018) A review on fundamental of high entropy alloys with promising high–temperature properties. J Alloys Compd 760:15–30. https://doi.org/10.1016/j.jallcom.2018.05.067
Kao Y-F, Lee T-D, Chen S-K, Chang Y-S (2010) Electrochemical passive properties of AlxCoCrFeNi (x=0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros Sci 52:1026–1034. https://doi.org/10.1016/j.corsci.2009.11.028
Zhang C, Chen GJ, Dai PQ (2016) Evolution of the microstructure and properties of laser-clad FeCrNiCoB x high-entropy alloy coatings. Mater Sci Technol 32:1666–1672. https://doi.org/10.1080/02670836.2015.1138035
Li J, Yang X, Zhu R, Zhang Y (2014) Corrosion and serration behaviors of TiZr0.5NbCr0.5VxMoy high entropy alloys in aqueous environments. Metals 4:597–608. https://doi.org/10.3390/met4040597
Slone CE, Miao J, George EP, Mills MJ (2019) Achieving ultra-high strength and ductility in equiatomic CrCoNi with partially recrystallized microstructures. Acta Mater 165:496–507. https://doi.org/10.1016/j.actamat.2018.12.015
Gludovatz B, George EP, Ritchie RO (2015) Processing, microstructure and mechanical properties of the CrMnFeCoNi high-entropy alloy. JOM 67:2262–2270. https://doi.org/10.1007/s11837-015-1589-z
Otto F, Dlouhý A, Somsen Ch et al (2013) The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater 61:5743–5755. https://doi.org/10.1016/j.actamat.2013.06.018
Gali A, George EP (2013) Tensile properties of high- and medium-entropy alloys. Intermetallics 39:74–78. https://doi.org/10.1016/j.intermet.2013.03.018
Stephan-Scherb C, Schulz W, Schneider M et al (2021) High-temperature oxidation in dry and humid atmospheres of the equiatomic CrMnFeCoNi and CrCoNi high- and medium-entropy alloys. Oxid Met 95:105–133. https://doi.org/10.1007/s11085-020-10014-7
Pešička J, Král R, Daniš S et al (2018) Structure and mechanical properties of FeAlCrV and FeAlCrMo medium-entropy alloys. Mater Sci Eng A 727:184–191. https://doi.org/10.1016/j.msea.2018.04.062
Sequeira CAC de (2019) High temperature corrosion: fundamentals and engineering, 1st edn. Wiley, Hoboken, NJ
Gulbransen EA, Andrew KF, Brassart FA (1963) Oxidation of molybdenum 550° to 1700 °C. J Electrochem Soc 110:952. https://doi.org/10.1149/1.2425918
Keller JG, Douglass DL (1991) The high-temperature oxidation behavior of vanadium-aluminum alloys. Oxid Met 36:439–464. https://doi.org/10.1007/BF01151591
Pradhan D, Mahobia GS, Chattopadhyay K, Singh V (2018) Severe hot corrosion of the superalloy IN718 in mixed salts of Na2SO4 and V2O5 at 700 °C. J Mater Eng Perform 27:4235–4243. https://doi.org/10.1007/s11665-018-3501-9
McCafferty E (2010) Introduction to corrosion science. Springer, New York, NY
Lu P, Saal JE, Olson GB et al (2019) Computational design and initial corrosion assessment of a series of non-equimolar high entropy alloys. Scr Mater 172:12–16. https://doi.org/10.1016/j.scriptamat.2019.07.003
Xiao DH, Zhou PF, Wu WQ et al (2017) Microstructure, mechanical and corrosion behaviors of AlCoCuFeNi-(Cr, Ti) high entropy alloys. Mater Des 116:438–447. https://doi.org/10.1016/j.matdes.2016.12.036
Lee CP, Chang CC, Chen YY et al (2008) Effect of the aluminium content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros Sci 50:2053–2060. https://doi.org/10.1016/j.corsci.2008.04.011
Chou YL, Yeh JW, Shih HC (2010) The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments. Corros Sci 52:2571–2581. https://doi.org/10.1016/j.corsci.2010.04.004
Esmaily M, Qiu Y, Bigdeli S et al (2020) High-temperature oxidation behaviour of AlxFeCrCoNi and AlTiVCr compositionally complex alloys. Npj Mater Degrad 4:25. https://doi.org/10.1038/s41529-020-00129-2
Schellert S, Gorr B, Laube S et al (2021) Oxidation mechanism of refractory high entropy alloys Ta-Mo-Cr-Ti-Al with varying Ta content. Corros Sci 192:109861. https://doi.org/10.1016/j.corsci.2021.109861
Gorr B, Azim M, Christ H-J et al (2015) Phase equilibria, microstructure, and high temperature oxidation resistance of novel refractory high-entropy alloys. J Alloys Compd 624:270–278. https://doi.org/10.1016/j.jallcom.2014.11.012
Engkvist J, Bexell U, Grehk M, Olsson M (2009) High temperature oxidation of FeCrAl-alloys—influence of Al-concentration on oxide layer characteristics: high temperature oxidation of FeCrAl-<!–alloys. Mater Corros 60:876–881. https://doi.org/10.1002/maco.200805186
Ham G-S, Kim Y-K, Na YS, Lee K-A (2021) Effect of Ti addition on the microstructure and high-temperature oxidation property of AlCoCrFeNi high-entropy alloy. Met Mater Int 27:156–165. https://doi.org/10.1007/s12540-020-00708-7
Shaburova NA, Moghaddam AO, Veselkov SN et al (2021) High-temperature oxidation behavior of AlxCoCrFeNiM (M = Cu, Ti, V) high-entropy alloys. Phys Mesomech 24:653–662. https://doi.org/10.1134/S1029959921060035
Bürckner M, Mengis L, White EMH, Galetz MC (2023) Influence of copper and aluminum substitution on high-temperature oxidation of the FeCoCrNiMn “Cantor” alloy. Mater Corros 74:79–90. https://doi.org/10.1002/maco.202213382
Ishii K, Kohno M, Ishikawa S, Satoh S (1997) Effect of rare-earth elements on high-temperature oxidation resistance of Fe–20Cr–5Al alloy foils. Mater Trans JIM 38:787–792. https://doi.org/10.2320/matertrans1989.38.787
Liu CM, Wang HM, Zhang SQ et al (2014) Microstructure and oxidation behavior of new refractory high entropy alloys. J Alloys Compd 583:162–169. https://doi.org/10.1016/j.jallcom.2013.08.102
Gorr B, Mueller F, Christ H-J et al (2016) High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb 20Mo 20Cr 20Ti 20Al with and without Si addition. J Alloys Compd 688:468–477. https://doi.org/10.1016/j.jallcom.2016.07.219
Acknowledgements
This work was supported by Czech Science Foundation under project GA21-03194S. E.J. acknowledges partial financial support from the Charles University Grant Agency under project number 278321. This publication was written with the support of the Institutional Endowment for the Long-Term Conceptual Development of Research Institutes, as provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2022.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This research did not contain any studies involving animal or human participants, nor did it take place on any private or protected areas.
Additional information
Handling Editor: Catalin Croitoru.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jača, E., Hotař, A., Pešička, J. et al. Oxidation properties of complex concentrated alloys FeAlCrV and FeAlCrMo. J Mater Sci 58, 9025–9037 (2023). https://doi.org/10.1007/s10853-023-08571-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08571-8