Abstract
Direct 3D bioprinting of bioreactors containing microorganisms embedded inside hydrogel structures is a promising strategy for biotechnological applications. Nevertheless, microporous hydrogel networks hinder the supply of nutrients and oxygen to the cell and limit cell migration and proliferation. To overcome this drawback, we developed a feedstock for 3D bioprinting structures with hierarchical porosity. The feedstock is based on a highly particle-filled alumina/alginate nanocomposite gel with immobilized E. coli bacteria with the protein ovalbumin acting as foaming agent. The foamed nanocomposite is shaped into a porous mesh structure by 3D printing. The pore radius diameters inside the non-printed, non-foamed nanocomposite structure are below 10 µm, between 10 and 500 µm in the albumin-stabilized foam and with additional pores in the range of 0.5 and 1 mm in the printed mesh structure. The influence of albumin on the bubbles and hence pore formation was analyzed by means of interfacial shear rheology and porosity measurements with X-ray microtomography (µCT). Furthermore, averaged diffusion coefficients of water in printed and non-printed samples with different albumin concentrations were recorded using nuclear magnetic resonance (NMR) tomography to assess the water content in the porous structure. Moreover, the effective viability and accessibility of embedded E. coli cells were analyzed for various material compositions. Here, the addition of albumin induced bacterial growth and the porosity increased the effective viability of the embedded bacteria, most likely because of enhanced accessibility of the cells. The experimental results demonstrate the potential of this approach for producing macroscopic bioactive materials with complex 3D geometries as a platform for novel applications in bioprocessing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of new hydrogel compositions and biocompatible cross-linking strategies opened the door to customize cell-containing materials into complex shapes by 3D bioprinting [1,2,3,4]. In order to maintain structural fidelity and mechanical properties, 3D bioprinting research has mainly utilized hydrogel structures with the main porosity stemming from the microporous polymer mesh. Although the used hydrogels are generally biocompatible, such comparably dense gels often hinder the supply of nutrients and oxygen to the cells [5,6,7] and limit the migration and proliferation of immobilized cells [8,9,10]. Consequently, there is a demand for hydrogel materials with hierarchical pore structures including larger interconnected pores that enable effective diffusion of nutrients/oxygen toward the material as well as cell proliferation. At the same time, the materials must exhibit good structural fidelity and adequate mechanical properties.
Hydrogel-based bioinks [11] are usually printed via extrusion of a viscoelastic feedstock followed by the deposition of the gel-like filaments and optionally a chemical cross-linking procedure. Both the printing process and the feedstock formulation must ensure high cell compatibility to avoid cell death during printing and cross-linking [12,13,14,15] while the viscoelastic properties of the feedstock need to be carefully tuned for optimal printing performance [16]. One of the most used natural hydrogels is alginate, which is a gelling polysaccharide with high biocompatibility that undergoes biocompatible ionotropic gelation with Ca2+ ions [17].
The majority of innovations in bioprinting have been achieved in the biomedical field [3, 18,19,20,21,22,23,24], but the use of bioprinting for bioprocessing using microorganisms is an emerging area which follows many of the same fundamental principles [25,26,27]. For example, Lehner et al. [25] printed a mixture of alginate and bacteria E. coli with rhamnose-inducible red fluorescent protein (RFP). They observed a reduction of bacterial viability of approx. 50% after printing but a change of color resulting from RFP could be observed from the immobilized bacteria. However, strategies using only alginate as support material are lacking in printability and shape fidelity. In this context, Wei et al. [28] showed a nanocomposite ink based on 2,2,6,6-tetramethylpiperidinyl-1-oxyl (TEMPO)-oxidized bacterial cellulose (TOBC), sodium alginate (SA), laponite nanoclay (Xls) and the protein BSA. By incorporating the nanoclay, they could improve printability while structural stability also increased. Furthermore, a reduction of swelling and dissolution in physiological media was observed for these nanocomposite materials. In our previous paper, we described the immobilization of bacteria E. coli and B. subtilis into rigid alginate/alumina nanocomposites by gel casting with good mechanical properties and high cell viability after 60 days of storage [29].
Ideally, 3D-printed structures with incorporated cells require a broad range of pores from nm to mm scales (hierarchical porosity). Small pores (nm scale) are essential for nutrient adsorption/retention as well as for structural support. Middle-sized pores (µm scale) are desired for cell proliferation as well as nutrient diffusion while pores in the mm scale decrease diffusion pathways and therefore enhance permeability of the material and accessibility of embedded cells [30, 31]. However, extrusion-based 3D printing of feedstocks that contain large pores is challenging due to potential nozzle blocking, strand breaks or poor shape fidelity [32, 33]. One strategy for printing porous filaments is to combine 3D printing and cryogelation and/or freeze-drying to produce filaments with approx. 10 µm pores [34]. Another strategy is to print porous filaments via the deposition of viscoelastic emulsions. Ying et al. [32] developed a porous bioink based on poly(ethylene oxide) (PEO) droplets dispersed in HepG2 cell/gelatin methacryloyl (GelMA) solution, followed by a removal of the PEO droplets to generate voids (pores) of 20–50 µm within the cross-linked GelMA phase. The enhanced porosity was demonstrated by the permeability with blue dextran while no permeability was observed in dense GelMA gels and the presence of the macropores resulted in improved cellular growth and viability. To the best of our knowledge, a bioprinting method that directly prints a viscoelastic foam has not been reported so far. In this context, Minas et al. [33] and Muth et al. [35] printed alumina wet foams and stabilized the bubbles with partially hydrophobized alumina particles, obtained by surface modification with the surfactants valeric acid and butyric acid, respectively. However, the presence of surfactant molecules and the low processing pH in these approaches are not ideal conditions for cell cultivation.
A detailed characterization of pore size, morphology, interconnectivity and its impact on fluid transport and diffusion within the material are crucial factors for designing porous materials [36]. Particularly modern X-ray computed tomography with submicrometer resolution (µCT) is an advanced imaging technique that has the ability to precisely visualize pore size/morphology as well as pore interconnectivity of different ceramic, metal and polymer materials [36, 37]. Complementary, nuclear magnetic resonance (NMR) tomography, among other things, is able to measure diffusion coefficients of water in porous materials in 3D space based on an evaluation of the volume-averaged diffusivity of the respective molecules. Thus, the combination of X-ray and NMR tomography techniques has the potential to provide a comprehensive picture of the inner structure of a porous material including both the material matrix and the contained fluid [38, 39].
In this study, we developed a feedstock for 3D bioprinting with hierarchical pore structure to minimize mass transport limitations in bioprocessing applications. The bioink is based on our previously developed feedstock which is a highly particle-filled bionanocomposite material containing alginate, alumina nanoparticles and E. coli bacteria [29]. To increase the porosity of the printed filaments and to create the hierarchical porosity, different concentrations of the protein ovalbumin were incorporated into the suspension as foaming agent and the influence of the protein on bulk and interfacial rheology as well as on the printability was analyzed. The porosity of the structure was characterized by µCT along with mercury intrusion porosimetry while the water content in the material based on its porosity and albumin concentration was evaluated by NMR tomography. Furthermore, the influence of the printing process and the material composition on bacterial viability was analyzed.
Materials and methods
Chemicals
Alumina powder was purchased from Almatis—Ludwigshafen am Rhein, Germany (CT 3000 SG, d50 = 500 nm, purity 99.78%). Sodium Alginate Protanal LFR 5/60 (Lot.: H192104) was obtained from FMC Biopolymer (Philadelphia, USA) and alginic acid sodium salt from brown algae, medium viscosity (product number: A2033), Glucose (product number: G8270), PBS (product number: P4417), lysogeny broth (LB) medium (product number: L3022), calcium chloride (product number: 208290) and protein albumin from chicken egg white powder 62–88% (ovalbumin) (product number: A5253) were purchased from Sigma-Aldrich Chemie GmbH—Munich, Germany. The assay to determine bacterial viability was based on the resazurin salt and purchased from Cayman chemical—Hamburg, Germany (product number: Cay14322).
Bacteria cultivation
The bacterial strain Escherichia coli K12 (DMS 1077) was obtained from Leibniz Institute DSMZ—Braunschweig, Germany, and the culture was set to grow overnight in sterile LB medium at 37 °C under agitation at 150 rpm in an incubator (Heidolph Unimax 1010—Schwalbach, Germany). Thereafter, cell suspension was centrifuged at 2500 rpm for 10 min to obtain a cell pellet. Then, the supernatant was discarded, and the bacteria pellet was resuspended with PBS until the desired concentration, by adjusting optical density at 595 nm, to approx. 32.5 × 108 cfu/mL of E. coli.
Feedstock preparation
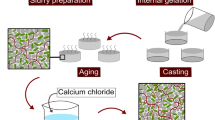
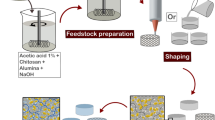
All colloidal dispersions were prepared under sterile conditions. First, a 4.2 wt. % alginate solution was prepared by dissolving 2.1 g alginate in 50 mL millipore water at room temperature (RT) via a dispermat (IKA RW20.n—Staufen, Germany) using a milk frother from Manufactum (Waltrop, Germany, product number: 27570) as stirring geometry for 30 min at 600 rpm. After total dissolution of alginate, alumina powder (66 vol. %) was slowly added into the alginate solution and was further stirred at 1200 rpm for 20 min for homogenization. Thereafter, the stirring velocity was decreased to 400 rpm and 11 mL of bacteria suspension in PBS was slowly added to the mixture, followed by intense stirring at 1000 rpm for 30 s. Afterward, different albumin powder concentrations were added into the suspensions, resulting in suspensions with final concentrations of 2, 4.5 and 7 wt% albumin. A feedstock without albumin was also produced for comparison. Successively, the suspensions with and without albumin were intensively stirred for two minutes at 1200 rpm with the milk frother stirrer for air incorporation as shown in Fig. 1. Subsequently, the samples were shaped as described below.
Scheme illustrating the bionanocomposite processing route based on ionotropic gelation. First alginate is dissolved in water followed by the addition of alumina powder and microorganisms to produce the feedstock. After homogenization of the mixture, ovalbumin is incorporated into the feedstock and a foam is produced by air incorporation. Thereafter, the suspensions are cast into the desired shape by either 3D printing or gel casting. Lastly, the samples are submersed in a calcium chloride solution for cross-linking
3D printing and gel casting
Biocomposite suspensions were printed with the 3D bioprinter Inkredible from Cellink—Gothenburg, Sweden, which is a pneumatic-based extrusion printer. First, 3 mL cartridges from Nordson (Erkrath, Germany) were filled with alginate/alumina suspensions with or without albumin. Lattice cuboids (1.5 × 1.5 × 0.5 mm) with a 90° grid structure were printed using conical precision tip nozzles (\(\varnothing\) 940 μm) from Techcon (Eastleigh Hampshire, UK) with an air pressure of 45 ± 5 kPa and a printing speed of 10 mm/s. The numerical code with the printing commands was generated using the Cellink Heartware 2.4.1 software, with a 67% infill density and 0.85 mm layer height, with exception of the first layer which had 0.7 mm height to assure a better contact between feedstock and substrate. Additionally, the printing settings were maintained constant during the whole printing procedure and were the same for all printed samples. Conversely, gel-cast samples were produced by pouring the suspension on a petri dish and slicing it into 2 × 1.5 × 0.5 mm cuboids. After shaping, gel-cast and printed samples were cross-linked for 8 min with a 0.5 M calcium chloride by dropping the solution onto the samples. Thereafter, samples were rigorously washed with PBS to stop the cross-linking.
Bulk rheology
Bulk rheological characterization of alginate/alumina feedstock with and without albumin was carried out in a stress-controlled rotational rheometer, Kinexus pro (Malvern Panalytical—Kassel, Germany) before cross-linking. Rheological tests were performed as follows: first, larger bubbles were mechanically removed from the fluids which were then deposited between the rheometer base plate and a \(\varnothing\) 20 mm parallel plate geometry at a gap of 0.5 mm and a temperature of 37 °C. All experiments were performed three times to assure reproducibility and using a solvent trap to avoid drying. Stepped shear rate increment experiments were performed to analyze the change of viscosity by varying shear rate range between 0.01 and 1000 s−1 with 8 logarithmic increments per decade. Additionally, the yield point of the suspensions was determined by a continuous shear rate ramp from 0.001 to 100 s−1. The shear viscosity was acquired using a stepped shear rate test at which each shear rate was held for one minute to allow for equilibration at each shear rate, while the yield point was determined using a shear rate ramp at which the shear rated is increased continuously. Thus, the first is a more steady-state test to comprehend feedstock behavior during the printing process and the second is a dynamic test to simulate the flow initiation. The shear rate ramp is a standard method to characterize a feedstock’s yield point while the stepped shear rate test is used to characterize fluid behavior such as Newtonian, shear thinning or shear thickening behavior [4]. Furthermore, shear rates inside the nozzle were calculated based on Blaeser et al. [40] and Balani et al. [41] and for both cases shear rates lower than 1000 s−1 were obtained.
Additionally, thixotropic behavior was analyzed by a three-step shear test with a step 1 at 0.05 s−1, step 2 at 50 s−1 and step 3 again at 0.05 s−1 and 60 s holding time for each step. Furthermore, oscillatory tests were performed to analyze the viscoelastic behavior of the suspensions with and without albumin. First amplitude sweep tests were performed between 0.01 and 100% oscillation amplitude (shear strain) with a constant frequency of 0.5 Hz to determine the linear-viscoelastic range. Thereafter, frequency sweep tests were performed from 0.01 to 50 Hz and 0.05% shear strain.
Interfacial rheology
Interfacial shear rheology properties were measured using a high-precision stress-controlled rheometer (DHR-3 rheometer from TA Instruments—New Castle, USA). First, alginate/alumina feedstocks with and without alumina were diluted ten times with water to ensure that the rheological modules of the bulk phase are significantly lower than those of the interface. Thereafter, 5.8 mL of the diluted suspension was loaded into a double-wall Delrin cup to pin the meniscus. Then, a Pt/Ir DuNoüy ring (20 mm ring diameter, 0.36 mm wire diameter) was positioned exactly at the air/suspension interface. Time test measurements were performed in the linear-viscoelastic region with a strain amplitude of 0.1% and frequency of 0.1 Hz. The measurements were repeated in triplicates showing similar plateau values, although the kinetics of adsorption were less reproducible. Solutions containing only water and albumin solution were tested as well for comparative purposes.
Feedstock printability characterization
Feedstock printability was evaluated by measuring the spacing between the interconnected channels in printed grid structure. Printed constructs should display a clear morphology with smooth surface, constant diameter after printing and be able to stack with other filaments without merging. Thus, squares holes should be formed between interconnected filaments for regular grids structures. Ouyang et al. [42] proposed an approach to define bioink printability (Pr) based on the shape of spacing between interconnected filaments for grids structures using the following function
where L means hole perimeter and A means hole area. For ideal printed grids, the interconnected channels of the constructs demonstrate a square shape and Pr = 1. When Pr > 1, printing constructs show irregular filaments with fractured morphology, usually due to an early cross-linking and for Pr < 1 more circular holes are formed due to filaments merging, caused by a liquid-like behavior of the feedstock. To determine Pr values of printed samples, grid structures were printed and the spacing between interconnected channels was analyzed after printing 3 and 8 layers with a digital optical microscope (VHX-5000 from Keyence—Neu-Isenburg, Germany) after drying at ambient conditions. Using microscope’s software ImageJ, perimeter and area of the space between the interconnected channels could be determined. Furthermore, cross section images were taken with the scanning electron microscopy (SEM) Camscan Series 2, from Obducat CamScan Ltd.—Cambridge, UK, with 80× magnification.
Mercury intrusion porosimetry
Sample porosity and pore size distribution were determined by mercury intrusion porosimetry (Pascal 140 and 440, Porotec—Hofheim am Taunus, Germany). Because this method is only able to detect pore sizes between 0.01 and 10 µm, we measured the porosity of samples without albumin. For that, printed samples were dried for 5 days at ambient conditions and then dry samples were analyzed.
X-ray micro-computed tomography (µ-CT)
To visualize the internal structure of the porous bodies and to quantify pores with sizes bigger than 10 µm, dry gel-cast and 3D-printed samples containing 2, 4.5 and 7 wt% of albumin as well as samples without albumin were cut into 5 × 5 × 5 mm cubes and were analyzed by X-ray micro-computed tomography (µ-CT) with a Xradia 520 Versa device (ZEISS—Jena, Germany). The tomography images are generated by the attenuation of X-rays passing through a specimen at the voltage and X-ray radiation strength specified in Table 1. Thereafter, a reconstruction algorithm based on the work of Katsevich et al. [43] is used to generate a 3D spatial representation based on the helix trajectory through which the object has moved along. In the reconstructed image, each voxel with its 16-bit grayscale value represents a corresponding X-ray attenuation coefficient that is associated with the density of the sample.
For the µCT measurements, a binary image was generated indicating either voids or the matrix by using the Otsu multilevel filter to segment the background of the investigated region of interest of the sample from the matrix and pore volumes. A morphological filter was applied on the segmented binary pore images to remove salt and pepper type noise with a kernel size of 3 voxels in each axis. Only pore diameter larger than 10 µm were resolved by µCT analysis and regions with smaller pore size appear as dense material in the filtered image. Connected pores were then separated by applying a distance transform filter on the binary image and subsequently the 3D watershed algorithm was applied. For pore analysis, each pore was labeled and their respective morphological parameters pore diameter, volume and sphericity were calculated. From the individual pore properties, the pore size and pore volume distributions were of pores > 10 µm were obtained as shown in Fig. 1. Additionally, to define sample total porosity we first calculate the total volume (VTotal) of the samples which is the sum of the solid part (Vsolid) and the volume of the pores (Vpore) derived from the µCT scans
The porosity of the samples with pores bigger than 10 µm (P>10) was defined as the quotient between Vpore and VTotal:
To quantify the pores smaller than 10 µm (P<10) in the complete ceramic structure, we multiplied the volume of the solid part Vsolid with the porosity results obtained from mercury intrusion porosimetry (PHg) and divided the product by VTotal.
The total porosity (Ptotal) is then the sum of P>10 and P<10.
NMR diffusion measurements
All magnetic resonance imaging (MRI) experiments were performed using a 7 Tesla preclinical MRI scanner (Bruker BioSpin MRI GmbH, Ettlingen, Germany) equipped with a magnetic field gradient system BGA12S2 (maximum gradient strength per direction (x,y,z) 442 mT/m, rise time 130 µs). The Software platform Paravision 5.1 was used for both MRI measurements and data processing. Gel-cast (GC) and 3D-printed samples with 0, 2, 4.5 and 7 wt% albumin were submerged in water for 2 weeks and thereafter positioned in the device. Images were generated using scout images of three orthogonal slices followed by a 3D gradient echo MRI sequence (repetition time TR = 30 ms, echo time TE = 0.9 ms, signal excitation with a flip angle of 30°, field-of-view (FOV): 32 × 32 × 32 mm3, matrix size: 128 × 128 × 128).
Bacterial viability test
The influence of alginate and albumin on bacterial viability was measured by incubating suspended bacteria in PBS with different concentrations of alginate (1, 0.5 and 0.25 wt%), with different concentrations of albumin (7, 3.5 and 1.75 wt%) and with different concentrations of alginate and albumin together (0.5 and 3.5 wt% alginate with 0.25 and 1.75 wt% albumin, respectively). Furthermore, control experiments with cells in PBS and in pure water were performed as well. Bacteria-containing suspensions were then incubated at 37 °C and 160 rpm and bacterial viability was determined after 4, 24 and 48 h of incubation in a PBS solution with 10% of resazurin stock solution. Resazurin stock solutions were produced as follows: 1 g of resazurin salt was dissolved in 100 mL of sterile PBS and stirred for homogenization, followed by a filtration step with 0.2 µm filters under sterile conditions to obtain resazurin stock solutions with a concentration of 10 g/L. Viable cells with active metabolism can reduce resazurin into resorufin, which is pink and fluorescent. The product was quantified by measuring the fluorescence at extinction at 540 nm and emission at 590 nm using a UV/vis photometer (Chameleon V from Hidex—Mainz, Germany).
The effective viability of immobilized bacteria was measured with an assay containing resazurin, as well. For that, three replicates of both gel-cast and 3D-printed samples containing E. coli with 0, 2, 4.5 and 7% albumin concentrations were incubated protected from light for 4 h at 37 °C and 160 rpm in a solution of PBS with 10% of resazurin solution stock, as described above. Thereafter, the bacterial viability was determined by measuring the fluorescence of the supernatant at extinction at 540 nm and emission at 590 nm. The same experiment was performed with different known concentrations of freely suspended bacteria and calibration curves were obtained to quantify the number of active cells in the bionanocomposites.
Results and discussion
Bulk rheology
The rheological properties are critical for controlling printability and shape fidelity of the feedstock formulations. In this study, we characterized the rheological properties of the feedstock to obtain information about its behavior before, during and after the printing process for all different concentrations of albumin. First, a shear rate ramp test was performed to analyze flow initiation and the material’s yield stress (Fig. 2a). The yield point of the feedstocks was approx. 690, 460, 400 and 220 Pa for samples with 0, 2, 4.5 and 7 wt% albumin, respectively. A sufficiently high yield point ensures that the material acts like a solid in steady state and only starts to flow after a suitable stress is applied.
Shear rate tests were performed to analyze the material’s flow behavior (Fig. 2b). All four compositions, with and without albumin, showed shear thinning behavior as a decrease in viscosity with increasing shear. A shear thinning behavior is a desirable characteristic for extrusion-based printing to ensure lower viscosity while the feedstock is pressed through the nozzle of the printing head at higher shear rates. Furthermore, all compositions show similar flow behavior which indicates that the addition of albumin concentrations did not significantly influence viscosity and flow behavior of the suspensions which is instead dominated by the interactions between alginate and alumina particles.
Post-printing recovery was estimated by a three-step thixotropy test by successively applying low (0.05 s−1), high (50 s−1) and low (0.05 s−1) shear rates, each for a duration of 60 s (Fig. 2c). All four feedstock compositions, with and without albumin, showed an initial viscosity of approx. 7800 Pa·s at 0.05 s−1 (base line). By increasing the shear rate to 50 s−1, the viscosity of all four feedstocks dropped to approx. 14 Pa·s. Subsequently, the shear rate was switched back to 0.05 s−1 and we observed the complete recovery of the initial viscosity. All feedstocks showed a high increase in viscosity to 550 Pa·s in the first two seconds and thereafter the viscosity continuously increased during 20 s until a plateau with the initial viscosity values was reached. Moreover, the shapes of the recovery curve of all four compositions are similar which again indicates that the addition of albumin did not significantly influence feedstock thixotropy.
Oscillatory rheology tests were used to characterize viscoelastic properties of the feedstocks by measuring the elastic (G′) and the viscous (G″) modulus. First, oscillatory deformation amplitude (strain) sweeps were performed with all four feedstocks compositions, with and without albumin (Fig. 2d). At low deformation amplitude, all samples showed solid-like behavior with G’ higher than G’’. This is already evident in the other gel-like properties discussed above, like high viscosity and a pronounced yield point. The gel-like behavior can be observed for all four compositions up to 10% shear strain. Afterward, G’’ is higher than G’, which manifests in a fluid-like behavior. Furthermore, frequency sweep tests were performed to observe internal changes in the material (Fig. 2e). All four compositions showed a gel-like behavior with constant moduli during the whole experiment. For both amplitude and frequency sweep tests, the addition of albumin at different concentrations did not significantly influence the rheological behavior of the feedstocks.
Interfacial shear rheology
Interfacial shear rheology experiments were performed to analyze the contributions of alginate, particles and proteins to the formation of interfacial films that lead to bubble stabilization. Figure 3 shows an overview of the two-dimensional storage modulus G’ as a function of time for water and feedstocks with albumin concentrations of 2 and 7 wt% and a control without albumin. G’’ is omitted for clarity and is much lower than G’ for all samples. Note that the suspensions were diluted ten times with water to ensure that rheological modules of the bulk phase are significantly lower than those at the interface. First, we analyzed the interfacial shear rheology of the feedstock with alumina particles but without albumin and the data showed a broad noise spectrum around 10–3 N/m. Accordingly, the device is not sensitive enough to detect potential adsorption of particles at the suspension/air interface. Furthermore, if particles did adsorb at the interface at all, most likely no cohesive thin film was formed [44].
When albumin is added into the feedstock, elastic moduli around 10–2 N/m are recorded, indicating that elastic thin films were formed at the interface. Moreover, a constant elastic modulus could be observed already at the beginning of the experiment, indicating fast film formation at the interface. Furthermore, the addition of different concentrations of albumin (2 and 7 wt%) did not seem to influence the elastic moduli of the film. This indicates that already with 2 wt% albumin the interface was saturated with albumin resulting in a stable and cohesive interfacial film. Additionally, albumin dissolved in water showed similar elastic moduli as the suspension containing albumin. This indicates that mostly albumin was responsible for the film formation and alginate or the particles did not significantly influence film formation.
Printability characterization
Next to rheological behavior, several other parameters can influence shape fidelity and integrity of the printed filaments. Here, feedstock printability (Pr) was assessed by measuring the spacing between printed constructs. Ideally, feedstocks (Pr = 1) should demonstrate a clear morphology with smooth surface and constant diameter of the extruded filament, which results in square holes in the fabricated regular grids. An irregular spacing (Pr > 1) could for example be observed due to irregular filaments with fractured morphology which might be the result of premature cross-linking or solidification, while a more liquid-like behavior feedstock can result in more circular spacing (Pr < 1) due to filament merging.
To test printability, all four feedstock compositions, with and without albumin, were printed through a ø 0.93 mm nozzle into a cuboid (final size 2 × 2 × 1 cm) with a 90° grid structure and printability was analyzed after printing 3 and 8 layers (Fig. 4). The printed filaments exhibited smooth surfaces and with the addition of albumin into the feedstock, small bubbles can be observed on the filament surface. The addition of albumin in either quantities (2, 4.5 or 7 wt%) maintained good feedstock printability even after printing 8 layers without significantly influencing printability despite the presence of the larger pores inside the strands. On the contrary, samples without albumin showed optimal printability values after printing 3 layers, but after printing 8 layers the structures began to sag and the holes could poorly be measured, indicating that samples without albumin could not resist the weight of following layers and collapsed. Square shapes between the printed channels were observed after printing 3 layers, with Pr values of 0.96 ± 0.02, 1.15 ± 0.05, 1.00 ± 0.05 and 1.03 ± 0.02 for samples with 0, 2, 4.5 and 7 wt% albumin, respectively. After printing 8 layers with 0, 2, 4.5 and 7 wt% albumin, the samples showed Pr values of 0.91 ± 0.03, 1.02 ± 0.04, 1.02 ± 0.02 and 1.02 ± 0.03, respectively. Interestingly, the Pr value of 2 wt% albumin after printing 3 layers is higher than with 4.5 and 7 wt% but the Pr values of samples containing albumin were all similar after printing 8 layers. Upon visual inspection, the samples at 2 wt% albumin show some irregularities in filament width, which are not clearly represented in the Pr values that only consider the sphericity of the structure. These irregularities might be related to the relatively low water content at 2 wt% albumin (see discussion below) and therefore increased weight of the extruded strands. The first printed layer was set to a lower height than the subsequent layers to assure a better contact between feedstock and substrate and this difference was not taken into account in the Pr value measurement. However, depending on the composition this interaction could be higher or lower. Thus, a higher Pr value of the 2 wt% albumin samples might indicate a higher interaction of the feedstock with the printing substrate (plastic petri dish), which decreased by increasing the albumin concentration, resulting in a more rectangular form of the spacings for the 2 wt% albumin feedstock. Nevertheless, this did not influence further printed layers. Furthermore, the circular forms of the printed filaments could be observed clearly in the vertical cross sections for samples containing albumin while for samples without albumin no filaments could be identified.
As discussed above, the printability of a feedstock can usually be described by its rheological behavior. Here, based on the rheological characterization, samples with and without albumin should exhibit similar printability but we observed a lower printability of samples without albumin after printing 3 and 8 layers. Consequently, the difference in printability between the samples may be due to different densities whereas filaments without albumin are heavier due to the absence of air bubbles.
Higher concentrations of albumin were not tested since no influence on porosity or on printability as well as on rheological properties could be observed but higher concentration might decrease material stability due to a lower cross-linking density of the printed filaments.
Sample porosity
Sample porosity was determined by a combination of two methods: mercury intrusion porosimetry and µCT scans. First, the porosity of samples with 0% albumin was measured by mercury intrusion porosimetry (Fig. 5) which is able to record pores with diameters between 0.01 and 10 µm (Fig. 5). Here, the samples showed 40% open porosity and pore size distribution with pores ranging from 0.01 to 0.2 µm.
Pores bigger than 10 µm were analyzed using µCT (Fig. 6). For 0% albumin samples, just a few pores bigger than 10 µm could be observed. A higher concentration of pores could be obtained for samples containing albumin with pore radius mainly ranging from 10 to 150 µm for gel-cast (GC) and 3D-printed (3D) samples with additional pore sizes ranging from 150 to 500 µm for 3D-printed samples stemming from the printed mesh structure. Note that here the pores obtained between the printed constructs were also considered into the pore size distribution. Moreover, a slight influence of the printing process on pore size distribution could be observed for samples containing albumin with a reduction of medium-sized pores (10–150 µm) in comparison with GC samples, which might be related to bubble coalescence during the printing process. Nevertheless, the printed samples still maintained most pores and exhibit high porosity as desired.
To determine total sample porosity, we combined both measurement techniques to be able to measure pores from 0.01 to 500 µm. For this purpose, we used the µCT scans to reconstruct digital representations of the samples from which the total volume was derived. For the regions with pore size smaller than 10 µm, which are shown as dense regions in the filtered µCT scans, porosity was obtained by the mercury intrusion measurements. Thus, the total porosity can be calculated by adding up the porosity of the apparently dense region in µCT and the porosity as determined by mercury intrusion porosimetry to the total material porosity (Table 2). The addition of 2 wt% albumin to the gel-cast samples increased the porosity > 10 µm by approx. 18%, in comparison with gel-cast samples without albumin, whereas the addition of 2 wt% albumin followed by 3D printing increased the porosity > 10 µm by approx. 32%. Further comparing 2 wt% albumin samples with gel-cast samples without albumin, sample total porosity increased approx. 10 and 20% for gel-cast and 3D printed. Moreover, higher concentrations of albumin did not significantly influence the total porosity further. The difference in porosity confirms the explanation for the difference of printability between samples containing albumin and without it, showing samples without albumin have lower porosity and therefore a higher density than samples containing albumin.
NMR diffusion characterization
The volume-averaged effective diffusion coefficient of water in the samples produced by gel casting (GC) and 3D printing (3D) with different concentrations of albumin was measured using NMR tomography. In this context, the effective diffusion coefficients provide a relative measure of water concentration in the sample and therefore help to assess changes in porosity in the wet hydrogel nanocomposite samples with different compositions. First, the diffusion coefficient of the liquid in the structure was determined by choosing three arbitrary regions of interest (ROIs). The averaged diffusion coefficients D were reported for the measured ROIs based on an exponential relation between signal amplitude and diffusion weighting S = S0 ∙ exp(−bD) with the signal amplitudes S and S0 measured with and without diffusion sensitizing gradients and the strength of diffusion weighting b. The data is given in Table 3. Additionally, Fig. 7 shows GC and 3D-printed samples containing different albumin concentrations with the obtained signal intensity versus the applied diffusion weighting (DW). For GC samples, the diffusion coefficient increased from 0.93 to 1.07, 1.24 and 1.24 103 mm2 s−1 while for 3D-printed samples, the diffusion coefficient increased from 0.63 to 1.03, 1.27 and 1.63 103 mm2 s−1 for samples with 0, 2, 4.5 and 7% albumin, respectively.
Contrary to the CT and mercury intrusion results, an increase in water content and therefore porosity was observed in samples with higher albumin concentration. This might be explained by the different sample preparation since CT and mercury intrusion measurements were performed with dry samples and NMR experiments with wet samples. Accordingly, slight swelling of the structure might have led to increased water uptake and an increase in porosity. Notably, albumin itself is capable of absorbing water and forming gels [45,46,47] and consequently a higher concentration of albumin might increase the water content of the sample. Furthermore, the printing process did not seem to have a significant influence on the diffusion coefficient compared to the gel-cast samples (Table 3).
Bacterial viability
Compatibility of the nanocomposite gels with bacterial cells was exemplarily characterized for E. coli. To determine the influence of the feedstock’s chemical composition and printing process on the embedded bacteria, we analyzed the effective viability of the immobilized bacteria in gel-cast and 3D-printed samples with all four compositions after calcium cross-linking by cellular reduction of resazurin (blue color) into resorufin (pink and fluorescent color). The effective bacteria viability was then quantified by the resazurin assay after 4, 24 and 48 h of incubation in PBS (Fig. 8a) and compared with the viability of the same quantity of freely suspended cells (Fig. 8b). It is important to note that the obtained effective bacterial viability relates to the viability of the fraction of cells embedded inside the samples that are accessible by resazurin molecules and the corresponding metabolites.
For gel-cast samples (GC), approx. 20% effective bacterial viability was measured with 0% and 2% albumin samples after 4 h of incubation. By increasing albumin concentrations to 4 and 7%, the effective bacterial viability also increased to 40 and 50%, respectively. This increase in effective bacterial viability is an interesting behavior since pore size and porosity minimally changed between the different albumin concentrations. Thus, to understand the effects of alginate and albumin on the viability of the accessible bacteria, analogous viability tests were performed after 4, 24 and 48 h of incubation with freely suspended cells and different concentrations of alginate (1, 0.5 and 0.25 wt%), different concentrations of albumin (7, 3.5 and 1.75 wt%) and with different concentrations of alginate and albumin mixtures (0.5 and 3.5 wt% and 0.25 and 1.75 wt%, respectively) (Fig. 8b). Alginate does not seem to influence viability of the suspended bacteria, since cells incubated with all alginate concentrations showed similar viability values as cells incubated in PBS. Conversely, an increase in bacterial viability could be observed incubating cells in albumin solutions, indicating that bacteria could use albumin proteins as nutrition, inducing bacterial growth. Furthermore, similar viability values could be observed for 7, 3.5 and 1.75 wt% albumin, as well as mixtures of alginate and albumin. Moreover, solutions containing 1.75% albumin and a mixture of 0.25% alginate and 1.75% albumin showed a decrease in cell viability after 24 and 48 h while no changes in bacterial viability were observed for the other samples containing 3.5 and 7% albumin. This may indicate that 1.75% albumin induced cell proliferation in the first hours, but the protein concentration was not sufficient to maintain cell viability after 24 and 48 h. Therefore, the slight increase in effective bacterial viability with increasing albumin concentration of gel-cast samples most likely indicates bacterial proliferation inside the structure.
Immobilized cells in 3D-printed samples (3D) with 0% albumin showed similar effective viability results (20%) as GC 0% albumin, indicating that the printing process has a minimal influence on the effective bacterial viability. Increasing albumin concentration results in an increase in effective viability of approx. 50, 80 and 120% after 4 h incubation for 2, 4.5 and 7% albumin samples, respectively. Thus, the effective bacterial viability of 3D-printed samples is significantly higher than for GC samples. This difference in bacterial viability is related to the higher accessibility of the embedded bacteria for the porous samples containing albumin while no significant differences between GC and 3D at 0% albumin could be observed, since the printed structure with 0% albumin collapsed to a similar shape as the GC sample. Furthermore, the effective viability over time shows a decrease in cell viability mainly for 4.5 and 7% albumin samples, which diverges from the freely suspended bacterial test (Fig. 8b). This may be related to the accessibility of the immobilized cells which may decrease over time due to cell growth and therefore cause a lower effective bacterial viability.
Conclusion
In summary, we developed a new feedstock suitable for 3D printing nanocomposite materials with hierarchical porosity and embedded bacteria. The feedstock is composed of a highly filled alginate/alumina nanocomposite to assure mechanical stability whereas the addition of ovalbumin as foaming agent added large pores in the range of 10–150 µm into the nanocomposite. The formation of interfacial films at the pore interface was analyzed by interfacial shear rheology to assess bubble stability. The results show that mostly albumin was responsible for film formation while alginate or the particles did not significantly stabilize the foam. Furthermore, rheological characterization of the bulk showed suitable characteristics for 3D printing like shear thinning, high yield stress, fast thixotropic recovery time and a gel-like behavior while albumin did not significantly influence bulk rheological properties. Nevertheless, the addition of albumin improved printability, which might be related to the difference in filament densities whereas filaments without albumin are heavier due to the absence of air bubbles. To quantify the sample’s porosity and pore size from 0.01 µm to 1 mm, a combination of mercury intrusion porosimetry and µ-CT was implemented. The addition of albumin increased the porosity by approx. 10 and 20% for gel-cast and 3D-printed samples, respectively, while different albumin concentrations did not significantly influence the porosity further in dry samples. Additionally, we observed with NMR tomography an increase in the water content with increasing albumin concentrations, which might be related to the capacity of albumin to form hydrogels. Furthermore, the influence of albumin addition could also be observed by analyzing the viability of E. coli bacteria, whereas a higher albumin concentration also resulted in higher effective cell viability. Furthermore, an increase in bacteria viability was observed with increasing albumin concentration in bacterial suspensions, indicating that bacteria could use albumin as nutrition. Consequently, the presence of albumin might enhance bacterial proliferation as well as accessibility of the bacteria inside the structure due to enhanced porosity. The open mesh structure of the printed parts further improved the accessibility of nutrients to the bacteria inside the structure. This demonstrates the potential of 3D-printed hierarchical structures for novel applications in bioprocessing and related applications.
References
Correia Carreira S, Begum R, Perriman AW (2020) 3D bioprinting: the emergence of programmable biodesign. Adv Healthc Mater 9(15):1900554
Skardal A, Atala A (2015) Biomaterials for integration with 3D bioprinting. Ann Biomed Eng 43:730
Tasoglu S, Demirci U (2013) Bioprinting for stem cell research. Trends Biotechnol 31:10
Dababneh AB, Ozbolat IT (2014) Bioprinting technology: a current state-of-the-art review. ASME J Manuf Sci Eng 136(6):061016
Willaert R (2007) Cell immobilization and its applications in biotechnology: current trends and future prospects. In: Fermentation microbiology and biotechnology, 2nd edn. CRC Press, pp 289–362
Nemati M, Webb C (2011) 2.24—Immobilized cell bioreactors. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Academic Press, Burlington, pp 331–346
Zhu Y (2007) Chapter 14—Immobilized cell fermentation for production of chemicals and fuels. In: Yang S-T (ed) Bioprocessing for value-added products from renewable resources. Elsevier, Amsterdam, pp 373–396
Armstrong JPK et al (2016) 3D bioprinting using a templated porous bioink. Adv Healthc Mater 5(14):1724–1730
Kessel B et al (2020) 3D bioprinting of macroporous materials based on entangled hydrogel microstrands. Adv Sci 7:2001419
Ruiz-Cantu L et al (2020) Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater Sci Eng: C 109:110578
Groll J et al (2018) A definition of bioinks and their distinction from biomaterial inks. Biofabrication 11(1):013001
Chimene D, Kaunas R, Gaharwar AK (2020) hydrogel bioink reinforcement for additive manufacturing: a focused review of emerging strategies. Adv Mater 32(1):1902026
Hölzl K et al (2016) Bioink properties before, during and after 3D bioprinting. Biofabrication 8(3):032002
Gungor-Ozkerim PS et al (2018) Bioinks for 3D bioprinting: an overview. Biomater Sci 6(5):915–946
Donderwinkel I, van Hest JCM, Cameron NR (2017) Bio-inks for 3D bioprinting: recent advances and future prospects. Polym Chem 8(31):4451–4471
Chimene D et al (2016) Advanced bioinks for 3D printing: a materials science perspective. Ann Biomed Eng 44(6):2090–2102
Pillay V et al (1998) Ionotropic gelation: encapsulation of indomethacin in calcium alginate gel discs. J Microencapsul 15(2):215–226
Matai I et al (2020) Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226:119536
Markstedt K et al (2015) 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromol 16:1489
Hsieh FY, Lin HH, Hsu SH (2015) 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 71:48
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32:773
Tasnim N et al (2018) 3D bioprinting stem cell derived tissues. Cell Mol Bioeng 11(4):219–240
Skardal A, Zhang J, Prestwich GD (2010) Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 31:6173
Cui X et al (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng 18:1304
Lehner BAE, Schmieden DT, Meyer AS (2017) A straightforward approach for 3D bacterial printing. ACS Synth Biol 6(7):1124–1130
Trampe E et al (2018) functionalized bioink with optical sensor nanoparticles for O2 imaging in 3D-bioprinted constructs. Adv Func Mater 28(45):1804411
Freyman MC et al (2020) 3D printing of living bacteria electrode. Nano Res 13(5):1318–1323
Wei J et al (2020) A 3D-printable TEMPO-oxidized bacterial cellulose/alginate hydrogel with enhanced stability via nanoclay incorporation. Carbohydr Polym 238:116207
Condi Mainardi J, Rezwan K, Maas M (2019) Embedding live bacteria in porous hydrogel/ceramic nanocomposites for bioprocessing applications. Bioprocess Biosyst Eng 42(7):1215–1224
Bao G et al (2020) Triggered micropore-forming bioprinting of porous viscoelastic hydrogels. Mater Horiz 7(9):2336–2347
Shao L et al (2020) Sacrificial microgel-laden bioink-enabled 3D bioprinting of mesoscale pore networks. Bio-Des Manuf 3(1):30–39
Ying G-L et al (2018) Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv Mater 30(50):1805460
Minas C et al (2016) 3D Printing of emulsions and foams into hierarchical porous ceramics. Adv Mater 28(45):9993–9999
Béduer A et al (2018) Additive manufacturing of hierarchical injectable scaffolds for tissue engineering. Acta Biomater 76:71–79
Muth JT et al (2017) Architected cellular ceramics with tailored stiffness via direct foam writing. Proc Natl Acad Sci 114(8):1832
Tariq F et al (2011) Characterization of hierarchical pore structures in ceramics using multiscale tomography. Acta Mater 59(5):2109–2120
Nakanishi K (2016) Porosity measurement. In: Klein L, Aparicio M, Jitianu A (eds) Handbook of sol-gel science and technology. Springer, Cham, pp 1–11
Robinson N, D’Agostino C (2020) NMR investigation into the influence of surface interactions on liquid diffusion in a mesoporous catalyst support. Top Catal 63(3):319–327
Di Tullio V, Capitani D, Proietti N (2018) Unilateral NMR to study water diffusion and absorption in stone-hydrogel systems. Microporous Mesoporous Mater 269:180–185
Blaeser A et al (2016) Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv Healthc Mater 5(3):326–333
Bakrani Balani S et al (2019) Influence of printing parameters on the stability of deposited beads in fused filament fabrication of poly(lactic) acid. Addit Manuf 25:112–121
Ouyang L et al (2016) Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 8(3):035020
Katsevich A (2002) Theoretically exact filtered backprojection-type inversion algorithm for spiral CT. SIAM J Appl Math 62(6):2012–2026
Maas M et al (2013) Towards the synthesis of hydroxyapatite/protein scaffolds with controlled porosities: bulk and interfacial shear rheology of a hydroxyapatite suspension with protein additives. J Colloid Interface Sci 407:529–535
Nojima T, Iyoda T (2018) Egg white-based strong hydrogel via ordered protein condensation. NPG Asia Mater 10(1):e460–e460
Rathna G, Li J, Gunasekaran S (2004) Functionally-modified egg white albumen hydrogels. Polym Int 53(12):1994–2000
Godiya CB, Kumar S, Xiao Y (2020) Amine functionalized egg albumin hydrogel with enhanced adsorption potential for diclofenac sodium in water. J Hazard Mater 393:122417
Acknowledgements
We would like to thank DFG Research Training Group GRK 1860, ‘Micro-, meso- and macroporous nonmetallic materials: fundamentals and applications’ (MIMENIMA) for funding.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Condi Mainardi, J., Bonini Demarchi, C., Mirdrikvand, M. et al. 3D bioprinting of hydrogel/ceramic composites with hierarchical porosity. J Mater Sci 57, 3662–3677 (2022). https://doi.org/10.1007/s10853-021-06829-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06829-7