Abstract
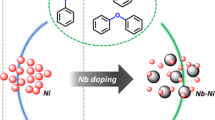
Nb-modified Mo–V–Nb tri-component oxides were synthesized for catalytic oxidative dehydrogenation of Cα–OH lignin model compounds. Nb–O octahedron as the nucleation center refined the Mo–V–Nb oxide particles with TiO2 as the support and changed the selective oxidation performance of the catalyst to a certain extent. However, the conversion of Cα–OH lignin model compounds was significantly reduced under the catalysis of Mo–V–Nb–O with too much Nb content. The experimental results showed that the effect of Nb in the three-component oxides not only reduced the crystal size of the catalyst, but also more importantly adjusted the valence state of vanadium. The valence state of vanadium in vanadyl octahedron formed based on Nb nucleation center was adjusted. The ratio between tetravalent vanadium and pentavalent vanadium was directly proportional to the conversion rate. The catalytic mechanism was that there were oxygen vacancies at the catalytic active sites of V4+, which provided a basis for the generation of reactive oxygen species in the oxidative dehydrogenation reaction.











Similar content being viewed by others
References
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Li C-Z, Zhao X-C, Wang A-Q et al (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624
Cao Y, Chen SS, Zhang S et al (2019) Advances in lignin valorization towards bio-based chemicals and fuels: lignin biorefinery. Bioresour Technol 291:121878
Yu X-N, Wei Z-Q, Lu Z-X et al (2019) Activation of lignin by selective oxidation: an emerging strategy for boosting lignin depolymerization to aromatics. Bioresour Technol 291:121885
Rajesh Banu J, Kavitha S, Yukesh Kannah R et al (2019) A review on biopolymer production via lignin valorization. Bioresour Technol 290:121790
Chen Y-G, Wang F, Jia Y-J et al (2017) One-step ethanolysis of lignin into small-molecular aromatic hydrocarbons over nano-SiC catalyst. Bioresour Technol 226:145–149
Ragauskas AJ, Beckham GT, Biddy MJ et al (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843
Xu C-P, Arancon R et al (2014) Lignin depolymerisation strategies: towards valuable chemicals and fuels. Chem Soc Rev 43:7485–7500
Sanderson K (2011) Lignocellulose: a chewy problem. Nature 474:S12–S14
Rahimi A, Azarpira A, Kim H et al (2013) Chemoselective metal-free aerobic alcohol oxidation in lignin. J Am Chem Soc 135:6415–6418
Lancefield CS, Stephen Ojo O et al (2015) Isolation of functionalized phenolic monomers through selective oxidation and C–O bond cleavage of the beta-O-4 linkages in lignin. Angew Chem Int Ed Engl 54:258–262
Tsai Y-T, Chen C-Y, Hsieh Y-J et al (2019) Selective Cα alcohol oxidation of lignin substrates featuring a β-O-4 linkage by a dinuclear oxovanadium catalyst via two-electron redox processes. Eur J Inorg Chem 2019:4637–4646
Gazi S (2019) Valorization of wood biomass-lignin via selective bond scission: a minireview. Appl Catal B Environ 257:117936
Tymchyshyn M, Yuan Z-S, Zhang Y-S et al (2019) Catalytic hydrodeoxygenation of guaiacol for organosolv lignin depolymerization—catalyst screening and experimental validation. Fuel 254:115664
Wang X-H, Wang N-N, Nguyen NN et al (2018) Catalytic depolymerization of lignin in ionic liquid using a continuous flow fixed-bed reaction system. Eng Chem Res 57:16995–17002
Hao Z-K, Li S-Y, Sun J-R et al (2018) Efficient visible-light-driven depolymerization of oxidized lignin to aromatics catalyzed by an iridium complex immobilized on mesocellular silica foams. Appl Catal B Environ 237:366–372
Zakzeski J, Bruijnincx P, Jongerius AL et al (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599
Rahimi A, Ulbrich A, Coon JJ et al (2014) Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515:249–252
Védrine JC (2019) Metal oxides in heterogeneous oxidation catalysis: state of the art and challenges for a more sustainable world. Chemsuschem 12:577–588
Zhang L-L, Hao K, Wang R-K et al (2020) Mo–V–Nb–O based catalysts for low temperature selective oxidation of Cα–OH lignin model compounds. Front Mater Sci. https://doi.org/10.1007/s11706-020-0494-8
Al-Mayman SI, Soliman MA et al (2018) Reaction kinetics of ethane partial oxidation to acetic acid. Appl Petrochem Res 8:29–38
Mishanin II, Kalenchuk AN, Maslakov KI et al (2017) Oxidative dehydrogenation of ethane over a Mo–V–Nb–Te–O mixed-oxide catalyst in a cyclic mode. Kinet Catal 58:156–160
Ishchenko EV, Gulyaev RV et al (2017) Effect of Bi on catalytic performance and stability of MoVTeNbO catalysts in oxidative dehydrogenation of ethane. Appl Catal A Gen 534:58–69
López-Medina R, Fierro JLG, Guerrero-Pérez MO et al (2011) Structural changes occurring at the surface of alumina-supported nanoscaled Mo–V–Nb–(Te)–O catalytic system during the selective oxidation of propane to acrylic acid. Appl Catal A Gen 406:34–42
Cheng M-J, Goddard WA (2015) In silico design of highly selective Mo–V–Te–Nb–O mixed metal oxide catalysts for ammoxidation and oxidative dehydrogenation of propane and ethane. J Am Chem Soc 137:13224–13227
Ishikawa S, Ueda W (2016) Microporous crystalline Mo–V mixed oxides for selective oxidations. Catal Sci Technol 6:617–629
Zhang L-L, Wang R-K, Song L et al (2019) Aerobic oxidative dehydrogenation of ethyl lactate over reduced MoVNbOx catalysts. Catal Lett 149:840–850
Li X-B, Iglesia E (2007) Synergistic effects of TiO2 and palladium-based cocatalysts on the selective oxidation of ethene to acetic acid on Mo–V–Nb oxide domains. Angew Chem Int Ed Engl 46:8649–8652
Li X-B, Iglesia E (2008) Kinetics and mechanism of ethane oxidation to acetic acid on catalysts based on Mo–V–Nb oxides. J Phys Chem C 112:15001–15008
Koltunov KY, Ishchenko EV, Sobolev VI (2018) Promoting effect of 4-dimethylaminopyridine on selective oxidation of benzyl alcohol over MoVTeNb mixed oxides. Catal Commun 117:49–52
Chu B-Z, An H, Chen X et al (2016) Phase-pure M1 MoVNbTeOx catalysts with tunable particle size for oxidative dehydrogenation of ethane. Appl Catal A Gen 524:56–65
Pacquette AL, Oh DS, Gewirth AA (2015) Mo–V–O based electrocatalysts for low temperature alcohol oxidation. J Phys Chem C 120:15553–15562
Chen X, Dang D, An H et al (2019) MnOx promoted phase-pure M1 MoVNbTe oxide for ethane oxidative dehydrogenation. J Taiwan Inst Chem E 95:103–111
Melzer D, Mestl G, Wanninger K et al (2019) Design and synthesis of highly active MoVTeNb-oxides for ethane oxidative dehydrogenation. Nat Commun 10:4012
Ziolek M, Sobczak I (2017) The role of niobium component in heterogeneous catalysts. Catal Today 285:211–225
Gao R-L, Li Y-D, Kim H et al (2018) Selective oxidation of lignin model compounds. Chemsuschem 11:2045–2050
Zhao X-Y, Zhang C-H, Xu C et al (2016) Kinetics study for the oxidative dehydrogenation of ethyl lactate to ethyl pyruvate over MoVNbOx based catalysts. Chem Eng J 296:217–224
Kardash TY, Plyasova LM, Bondareva VM et al (2010) M5O14-like V–Mo–Nb oxide catalysts: structure and catalytic performance. Appl Catal A Gen 375:26–36
Jones R, Adams JM, Evans S (1987) A new barium molybdate phase. Mater Res Bull 22:351–358
Ziolek M, Sobczak I, Decyk P et al (2015) Search for reactive intermediates in catalytic oxidation with hydrogen peroxide over amorphous niobium(V) and tantalum(V) oxides. Appl Catal B: Environ 164:288–296
Al-mashta F, Sheppard N, Lorenzelli V et al (1982) Infrared study of adsorption on oxygen-covered α-Fe2O3: bands due to adsorbed oxygen and their modification by co-adsorbed hydrogen or water. J Chem Soc, Faraday Trans 1(78):979–989
Che M, Tench AJ (1983) Characterization and reactivity of molecular oxygen species on oxide surfaces. Adv Catal 32:1–148
Rosen GM, Beselman A, Tsai P et al (2004) Influence of conformation on the EPR spectrum of 5,5-dimethyl-1-hydroperoxy-1-pyrrolidinyloxyl: a spin trapped adduct of superoxide. J Org Chem 69:1321–1330
Diaz-Uribe CE, Daza MC, Martínez F et al (2010) Visible light superoxide radical anion generation by tetra(4-carboxyphenyl)porphyrin/TiO2: EPR characterization. J Photochem Photobiol A 215:172–178
Dikalov S, Jiang JJ, Mason RP (2005) Characterization of the high-resolution ESR spectra of superoxide radical adducts of 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide (DEPMPO) and 5,5-dimethyl-1-pyrroline N-oxide (DMPO). Analysis of conformational exchange. Free Radical Res 39:825–836
Yamada M, Karlin KD, Fukuzumi S (2016) One-step selective hydroxylation of benzene to phenol with hydrogen peroxide catalysed by copper complexes incorporated into mesoporous silica–alumina. Chem Sci 7:2856–2863
Ziolek M, Sobczak I, Lewandowska A et al (2001) Oxidative properties of niobium-containing mesoporous silica catalysts. Catal Today 70:169–181
Ziolek M, Sobczak I, Nowak I et al (2000) Nb-containing mesoporous molecular sieves—a possible application in the catalytic processes. Microporous Mesoporous Mater 35–36:195–207
Acknowledgement
This work was supported by the National Natural Science Foundation of China (Grant No. 21676285); the Qingdao Indigenous Innovation Program (Grant No. 15-9-1-76-jch); and the Scientific Research Foundation of Shandong University of Science and Technology for Recruited Talents (Grant No. 2017RCJJ015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hao, K., Zhang, LL., Song, L. et al. Effect of Nb on catalyst nanoparticle sizes and catalytic activities of H2O2-mediated oxidative dehydrogenation of Cα–OH lignin model compounds. J Mater Sci 55, 10492–10504 (2020). https://doi.org/10.1007/s10853-020-04783-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04783-4