Abstract
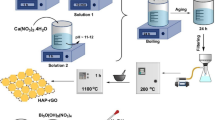
Doping tungsten oxide with other metals is a promising approach for obtaining improved properties as compared with its undoped counterpart. Here, the effect of molybdenum doping on the morphology, crystalline structure, and adsorption properties of nanostructured WO3 was investigated. Undoped and Mo-doped hexagonal WO3 (h-WO3) nanowire bundles were synthesized by hydrothermal method. The samples were characterized by X-ray powder diffraction, transmission electron microscopy, scanning transmission electron microscopy, selected-area electron diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy, and Raman spectroscopy. The surface areas of the samples were evaluated by N2 adsorption–desorption isotherm, and their adsorption properties were evaluated based on their ability to adsorb cationic dye methylene blue (MB) in aqueous solution. Upon the addition of molybdenum, the morphology of the h-WO3 nanowire changes from large bundles to narrow bundles with a higher number of isolated thin nanowires. Mo doping also has changed the crystalline structure, porosity, and surface area of the bundled h-WO3 nanowires. Interestingly, Mo-doped h-WO3 nanowire bundles exhibit an enhanced and faster MB uptake, thus implying that Mo-doping WO3 can be an effective strategy to improve the adsorption properties, thereby adding value in developing alternative adsorption-based methods for wastewater treatment.





Similar content being viewed by others
References
Field MS, Wilhelm RG, Quinlan JF, Aley TJ (1995) An assessment of the potential adverse properties of fluorescent tracer dyes used for groundwater tracing. Environ Monit Assess 38:75–96
Sharma MK, Sobti RC (2000) Rec effect of certain textile dyes in Bacillus subtilis. Mutat Res Genet Toxicol Environ Mutagen 465:27–38
Katheresan V, Kansedo J, Lau SY (2018) Efficiency of various recent wastewater dye removal methods: a review. J Environ Chem Eng 6:4676–4697
Al-Ghouti MA, Khraisheh MAM, Allen SJ, Ahmad MN (2003) The removal of dyes from textile wastewater: a study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J Environ Manag 69:229–238
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184
Singh K, Arora S (2011) Removal of synthetic textile dyes from wastewaters: a critical review on present treatment technologies. Crit Rev Environ Sci Technol 41:807–878
Al-Degs Y, Khraisheh MAM, Allen SJ et al (2007) Competitive adsorption of reactive dyes from solution: equilibrium isotherm studies in single and multisolute systems. Chem Eng J 128:163–167
Li J, Liu X, Han Q et al (2013) Formation of WO3 nanotube-based bundles directed by NaHSO4 and its application in water treatment. J Mater Chem A 1:1246–1253
Chen H, He J (2008) Facile synthesis of monodisperse manganese oxide nanostructures and their application in water treatment. J Phys Chem C 112:17540–17545
Sharma YC, Srivastava V, Singh VK et al (2012) Nano-adsorbents for the removal of metallic pollutants from water and wastewater. Environ Technol 30:37–41
Hu J, Shipley HJ (2012) Evaluation of desorption of Pb (II), Cu (II) and Zn (II) from titanium dioxide nanoparticles. Sci Total Environ 431:209–220
Wang J, Liu B, Wu J, Li H (2018) Crystallization of WO3·H2O nanosheets with high-adsorption capacity for methylene blue. J Nanoparticle Res 20:253
Li H, Li J, Hou C et al (2017) Solution-processed porous tungsten molybdenum oxide electrodes for energy storage smart windows. Adv Mater Technol 2:2–7
Li H, McRae L, Firby CJ et al (2018) Nanohybridization of molybdenum oxide with tungsten molybdenum oxide nanowires for solution-processed fully reversible switching of energy storing smart windows. Nano Energy 47:130–139
Chang K, Hu C, Huang C et al (2011) Microwave-assisted hydrothermal synthesis of crystalline WO3–WO3·0.5H2O mixtures for pseudocapacitors of the asymmetric type. J Power Sources 196:2387–2392
Li M, Li W, Liu S (2011) Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr Res 346:999–1004
Kumagai N, Kumagai N, Umetzu Y et al (1996) Synthesis of hexagonal form of tungsten trioxide and electrochemical lithium insertion into the trioxide. Solid State Ion 86–88:1443–1449
Gong Y, Zheng F, Dong J et al (2019) Structural changes in hexagonal WO3 under high pressure. J Alloys Compd 797:1013–1017
Figlarz M (1989) New oxides in the WO3–MoO3 system. Prog Solid State Chem 19:1–46
Nayak AK, Lee S, Choi YI et al (2017) Crystal phase and size-controlled synthesis of tungsten trioxide hydrate nanoplates at room temperature: enhanced Cr(VI) photoreduction and methylene blue adsorption properties. ACS Sustain Chem Eng 5:2741–2750
Zhang S, Yang H, Huang H et al (2017) Unexpected ultrafast and high adsorption capacity of oxygen vacancy-rich WOX/C nanowire networks for aqueous Pb2+ and methylene blue removal. J Mater Chem A 5:15913–15922
Xie S, Bi Z, Chen Y et al (2018) Electrodeposited Mo-doped WO3 film with large optical modulation and high areal capacitance toward electrochromic energy-storage applications. Appl Surf Sci 459:774–781
De León JMOR, Acosta DR, Pal U, Castañeda L (2011) Improving electrochromic behavior of spray pyrolised WO3 thin solid films by Mo doping. Electrochim Acta 56:2599–2605
Kalanur SS, Seo H (2018) Influence of molybdenum doping on the structural, optical and electronic properties of WO3 for improved solar water splitting. J Colloid Interface Sci 509:440–447
Wang F, Di Valentin C, Pacchioni G (2012) Doping of WO3 for photocatalytic water splitting: hints from density functional theory. J Phys Chem C 116:8901–8909
Zhang J, Lu H, Lu H et al (2019) Porous bimetallic Mo–W oxide nanofibers fabricated by electrospinning with enhanced acetone sensing performances. J Alloys Compd 779:531–542
Sun Y, Chen L, Wang Y et al (2017) Synthesis of MoO3/WO3 composite nanostructures for highly sensitive ethanol and acetone detection. J Mater Sci 52:1561–1572. https://doi.org/10.1007/s10853-016-0450-2
Song XC, Yang E, Liu G et al (2010) Preparation and photocatalytic activity of Mo-doped WO3 nanowires. J Nanopart Res 12:2813–2819
Du H, Yang C, Pu W et al (2019) Highly active Sb2S3-attached Mo–WO3 composite film for enhanced photoelectrocatalytic water splitting at extremely low input light energy. ACS Sustain Chem Eng 7:9172–9181
Silveira JV, Moura JVB, Luz-Lima C et al (2018) Laser-induced thermal effects in hexagonal MoO3 nanorods. Vib Spectrosc 98:145–151
Moura JVB, Silveira JV, da Silva Filho JG et al (2018) Temperature-induced phase transition in h-MoO3: stability loss mechanism uncovered by Raman spectroscopy and DFT calculations. Vib Spectrosc 98:98–104
Huang R, Shen Y, Zhao L, Yan M (2012) Effect of hydrothermal temperature on structure and photochromic properties of WO3 powder. Adv Powder Technol 23:211–214
Ghosh K, Roy A, Tripathi S et al (2017) Insights into nucleation, growth and phase selection of WO3: Morphology control and electrochromic properties. J Mater Chem C 5:7307–7316
Daniel MF, Desbat B, Lassegues JC et al (1987) Infrared and Raman study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide tydrates. J Solid State Chem 67:235–247
Chatzikyriakou D, Krins N, Gilbert B et al (2014) Mesoporous amorphous tungsten oxide electrochromic films: A Raman analysis of their good switching behavior. Electrochim Acta 137:75–82
Djaoued Y, Priya S, Balaji S (2008) Low temperature synthesis of nanocrystalline WO3 films by sol–gel process. J Non Cryst Solids 354:673–679
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl Chem 87:1051–1069
Yousefi R, Jamali-sheini F, Cheraghizade M (2015) Materials science in semiconductor processing enhanced visible-light photocatalytic activity of strontium-doped zinc oxide nanoparticles. Mater Sci Semicond Process 32:152–159
Akhtar MJ, Alhadlaq HA, Alshamsan A, Khan MAM (2015) Aluminum doping tunes band gap energy level as well as oxidative stress-mediated cytotoxicity of ZnO nanoparticles in MCF-7 cells. Sci Rep 5:13876(1)–138761(6)
Salem M, Akir S, Ghrib T et al (2016) Fe-doping effect on the photoelectrochemical properties enhancement of ZnO films Fe-doping effect on the photoelectrochemical properties enhancement of ZnO films. J Alloys Compd 685:107–113
Wang Y, Cheng J, Yu S et al (2016) Synergistic effect of N-decorated and Mn2+ doped ZnO nanofibers with enhanced photocatalytic activity. Sci Rep 6:32711(1)–32711(10)
Yu W, Yang LH, Teng XY et al (2013) Influence of structure characteristics on room temperature ferromagnetism of Ni-doped ZnO thin films. J Appl Phys 103:093901(1)–093901(4)
Majeed Khan MA, Kumar S, Naziruddin Khan M et al (2014) Microstructure and blueshift in optical band gap of nanocrystalline AlxZn1−xO thin films. J Lumin 155:275–281
Acknowledgements
JVS acknowledges the financial support of FUNCAP (grant no. 03/2018 BPI/Funcap). PTCF acknowledges PRONEX FUNCAP-CNPq (PR2-0101-00006.01.00/15). AGSF acknowledges support from CNPq (Grant 309309/2017-4) and CAPES Procad (88887.124162/2014-00). TLV acknowledges FAPERJ and CNPq. CLL acknowledges support from CNPq (Grants 311115/2017-9 and 422125/2018-0)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silveira, J.V., Moraes, E.C., Moura, J.V.B. et al. Mo-doped WO3 nanowires for adsorbing methylene blue dye from wastewater. J Mater Sci 55, 6429–6440 (2020). https://doi.org/10.1007/s10853-020-04472-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04472-2