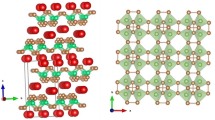
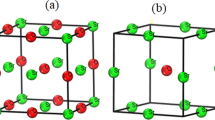
Abstract
The isomorphous substitution of \(\hbox {Al}^{3+}\) and/or \(\hbox {Fe}^{3+}\) cations into the erionite zeolite framework were studied through periodic density functional theory computations. Taking as reference the Mulliken charges for siliceous framework computed in previous work (Antúnez-García et al. in J Mol Struct 1059:232–238, 2014), the present results show that the basicity of ERI zeolite increases when those cations are introduced into the framework. It was also observed that when at least two \(\hbox {Si}^{4+}\) cations are isomorphically substituted by \(\hbox {Fe}^{3+}\) in the unit cell of the erionite zeolite, it acquires magnetic properties. The results show that a specific net-spin polarization (up or down) could be correlated with particular configurations of erionite zeolite, containing ferric iron.








Similar content being viewed by others
References
Breck DW (1974) Zeolite molecular sieves: structure, chemistry and use, 3rd edn. Willey, New York
Vansant E (1988) Pore size engineering in zeolites. Stud Surf Sci Catal 37:143–153
McCusker LB, Olson DH (2007) Atlas of zeolite framework types, 6th edn. Elsevier, Amsterdam
Breck DW, Smith JV (1959) Molecular sieves. Sci Am 200:85–96
Jae J, Tompsett GA, Foster AJ, Hammond KD, Auerbach SM, Lobo RF, Huber GW (2011) Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J Catal 279:257–268
Shi J, Wang Y, Yang W, Tang Y, Xie Z (2015) Recent advances of pore system construction in zeolite-catalyzed chemical industry processes. Chem Soc Rev 44:8877–8903
Townsend RP, Coker EN (2001) In: van Bekkum H, Flanigen E, Jacobs P, Jansen J (eds) Introduction to zeolite science and practice, vol 137. Studies in surface science and catalysis. Elsevier, Amsterdam, pp 467–524
Busca G (2017) Acidity and basicity of zeolites: a fundamental approach. Microporous Mesoporous Mater 254:3–16
Heidler R, Janssens GO, Mortier WJ, Schoonheydt RA (1996) Charge sensitivity analysis of intrinsic basicity of faujasite-type zeolites using the Electronegativity Equalization Method (EEM). J Phys Chem 100:19728–19734
Barthomeuf D, Mallmann AD (1988) Basicity and electronegativity of zeolites. Stud Surf Sci Catal 37:365–374
Barthomeuf D (2003) Framework induced basicity in zeolites. Microporous Mesoporous Mater 66:1–14
Yang C, He N, Xu Q (1999) Effect of trivalent elements in the framework on the basicity of zeolites. Stud Surf Sci Catal 125:457–464
Kumar R, Ratnasamy P (1991) Synthesis and characterisation of ferrisilicate zeolites. Stud Surf Sci Catal 60:43–52
Ratnasamy P, Kumar R (1991) Ferrisilicate analogs of zeolites. Catal Today 9:329–416
Meng L, Zhu X, Mezari B, Pestman R, Wannapakdee W, Hensen EJM (2017) On the role of acidity in bulk and nanosheet [T]MFI (T=\({\text{ Al }}^{3+}\), \({\text{ Ga }}^{3+}\), \({\text{ Fe }}^{3+}\), \({\text{ B }}^{3+}\)) zeolites in the methanol-to-hydrocarbons reaction. Chem Cat Chem 23:3942–3954
Jiang X, Su X, Bai X, Li Y, Yang L, Zhang K, Zhang Y, Liu Y, Wu W (2018) Conversion of methanol to light olefins over nanosized [Fe, Al]ZSM-5 zeolites: influence of Fe incorporated into the framework on the acidity and catalytic performance. Microporous Mesoporous Mater 263:243–250
Diallo MM, Laforge S, Pouilloux Y, Mijoin J (2018) Highly efficient dehydration of glycerol to acrolein over isomorphously substituted Fe-MFI zeolites. Catal Lett 148:2283–2303
Naraki Y, Ariga K, Oka H, Kurashige H, Sano T (2018) An isomorphously substituted Fe-BEA zeolite with high Fe content: facile synthesis and characterization. J Nanosci Nanotechnol 18:11–19
Bogomolov VN, Zadorozhnii AI, Panina LK, Petranovskii VP (1980) Transition of 13-atom, mercury clusters to a strongly paramagnetic state under the action of magnetic field. JETP Lett 31:339–342
Pierella LB, Saux C, Caglieri SC, Bertorello HR, Berco PG (2008) Catalytic activity and magnetic properties of Co-ZSM-5 zeolites prepared by different methods. Appl Catal A 347:55–61
Antúnez-García J, Posada-Amarillas A, Galván DH, Smolentseva E, Petranovskii V, Fuentes Moyado S (2016) DFT study of composites formed by \({\text{ M }}_{2}\) metallic clusters (\({\text{ M }}={\text{ Ni }}\), Cu, Fe and Cu) in faujasite. RSC Adv 6:79160–79165
Rao BK, Jena P (1985) Physics of small metal clusters: topology, magnetism, and electronic structure. Phys Rev B 32:2058–2069
Nozue Y, Kodaira T, Goto T (1992) Ferromagnetism of potassium clusters incorporated into zeolite LTA. Phys Rev Lett 68:3789–3792
Nozue Y, Kodaira T, Goto T (1992) Ferromagnetic properties of potassium clusters incorporated into zeolite LTA. Phys Rev Lett 48:12253–12261
Kelly MJ (1995) A model electronic structure for metal-intercalated zeolites. J Phys Condens Matter 7:5507–5519
Nakano T, Ikemoto Y, Nozue Y (1999) Ferromagnetism and paramagnetism in potassium clusters incorporated in zeolite LTA. Eur Phys J D 9:505–508
Hanh DT, Nakano T, Nozue Y (2010) Strong dependence of ferrimagnetic properties on Na concentration in Na-K alloy clusters incorporated in low-silica X zeolite. J Phys Chem Solids 71:677–680
Igarashi M, Nakano T, Thi PT, Nozue Y, Goto A, Hashi K, Ohki S, Shimizu T, Krajnc ACV, Jeglič P, Arčon D (2013) NMR study of thermally activated paramagnetism in metallic low-silica X zeolite filled with sodium atoms. Phys Rev B 87:075138. https://doi.org/10.1103/PhysRevB.87.075138
Kien LM, Goto T, Hanh DT, Nakano T, Nozue Y (2015) Ferromagnetism of Na–K alloy clusters incorporated in zeolite low-silica X. J Phys Soc Jpn 84:064718. https://doi.org/10.7566/JPSJ.84.064718
Nakano T, Nozue Y (2017) Electrons of alkali metals in regular nanospaces of zeolites. Adv Phys X 2:254–280
Johnson M, Silsbee RH (1985) Interfacial charge-spin coupling: injection and detection of spin magnetization in metals. Phys Rev Lett 55:1790–1793
Wolf SA, Awschalom DD, Buhrman RA, Daughton JM, von Molnár S, Roukes ML, Chtchelkanova AY, Treger DM (2001) Spintronics: a spin-based electronics vision for the future. Science 294:1488–1495
Wolf SA, Chtchelkanova AY, Treger DM (2006) Spintronics: a retrospective and perspective. IBM J Res Dev 50:101–110
Kostyleva MP, Serga AA, Schneider T, Leven B, Hillebrands B (2005) Spin-wave logical gates. Appl Phys Lett 87:153501. https://doi.org/10.1063/1.2089147
Gershenfeld NA, Chuang IL (1997) Bulk spin-resonance quantum computation. Science 275:350–356
Khajetoorians AA, Wiebe J, Chilian B, Wiesendanger R (2011) Realizing all-spinbased logic operations atom by atom. Science 332:1062–1064
Attanoos RL, Churg A, Galateau-Salle F, Gibbs AR, Roggli VL (2018) Malignant mesothelioma and its non-asbestos causes. Arch Pathol Lab Med 142:753–760
Emri SA (2017) The Cappadocia mesothelioma epidemic: its influence in Turkey and abroad. Ann Transl Med. https://doi.org/10.21037/atm.2017.04.06
Klyueva NV, Tien ND, Ione KG (1985) Hydrocarbon synthesis from methanol on erionite and mordenite catalysts synthesized in the presence of \({\text{ B }}^{3+}\), \({\text{ Ga }}^{3+}\) or \({\text{ Fe }}^{3+}\). React Kinet Catal Lett 29:427–432
Gualtieri AF, Gandol NB, Pollastri S, Pollok K, Langenhorst F (2016) Where is iron in erionite? A multidisciplinary study on fibrous erionite-Na from Jersey (Nevada, USA). Sci Rep 6:37981. https://doi.org/10.1038/srep37981
Roque-Malherbe R, Díaz-Aguila C, Reguera-Ruíz E, Fundora-Lliteras J, López-Colado L, Hernández-Vélez M (1990) The state of iron in natural zeolites: a Mössbauer study. Zeolites 10:685–689
Saini-Eidukat B, Triplett JW (2014) Erionite and offretite from the Killdeer Mountains, Dunn County, North Dakota, USA. Am Miner 99:8–15
Derouane EG, Vedrine JC, Pinto RR, Borges PM, Costa L, Lemos M, Lemos F, Ribeiro FR (2013) The acidity of zeolites: concepts, measurements and relation to catalysis: a review on experimental and theoretical methods for the study of zeolite acidity. Catal Rev Sci Eng 55:454–515
Davis RJ (2000) Relationships between basicity and reactivity of zeolite catalysts. Res Chem Intermed 26:21–27
Chen F, Zhang L, Feng G, Wang X, Zhang R, Liu J (2018) Trivalent ions modification for high-silica mordenite: a first principles study. Appl Surf Sci 433:627–638
Nicholas J (1997) Density functional theory studies of zeolite structure, acidity, and reactivity. Topics Catal 4:157–171
Zhou D, Bao Y, Yang M, He N, Yang G (2006) DFT studies on the location and acid strength of Brönsted acid sites in MCM-22 zeolite. J Mol Catal A 244:11–19
Liu C, Traca I, van Santen RA, Hensen EJM, Pidko EA (2017) Scaling relations for acidity and reactivity of zeolites. J Phys Chem C Nanomater Interfaces 121:23520–23530
Niwa M, Sususki K, Morishita N, Sastre G, Okumura K, Katada N (2013) Dependence of cracking activity on the Brøsted acidity of Y zeolite: DFT study and experimental confirmation. Catal Sci Technol 3:1919–1927
Antúnez-García J, Galván DH, Posada-Amarillas A, Petranovskii V (2014) A theoretical study of Cu clusters in siliceous erionite. J Mol Struct 1059:232–238
Löwenstein S (1954) The distribution of aluminum in the tetrahedra of silicates and aluminates. Am Miner 39:92–96
Antúnez-García J, Galván DH, Petranovskii V, Posada-Amarillas A (2015) A DFT study of copper-oxide clusters embedded in dry and water-immerse mordenite. Comput Mater Sci 106:140–148
Deka RC, Roy RK, Hirao K (2000) Basicity of the framework oxygen atom of alkali and alkaline earth-exchanged zeolites: a hard-soft acid-base approach. Chem Phys Lett 332:576–582
Barthomeuf D (2006) Basic zeolites: characterization and uses in adsorption and catalysis. Catal Rev Sci Eng 38:521–612
Bandyopadhyay S, Cahay M (2009) Electron spin for classical information processing: a brief survey of spin-based logic devices, gates and circuits. Nanotechnology 20:412001. https://doi.org/10.1088/0957-4484/20/41/412001
Acknowledgements
This research was supported by the Project SENER-CONACyT 117373, UNAM PAPIIT IN107817 Grant and RFBR-CITMA Project No. 18-53-34004 and through the basic-science proposal A1-S-33492. We also want to thank for the supercomputing time provided by the UNAM through the project LANCAD-UNAM-DGTIC-041.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Antúnez-García, J., Galván, D.H., Petranovskii, V. et al. Theoretical study of the effect of isomorphous substitution by \(\hbox {Al}^{3+}\) and/or \(\hbox {Fe}^{3+}\) cations to tetrahedral positions in the framework of a zeolite with erionite topology. J Mater Sci 54, 13190–13199 (2019). https://doi.org/10.1007/s10853-019-03845-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03845-6