Abstract
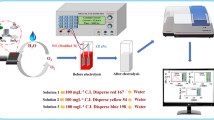
The titanium-based electrodes with MnOx nanoparticles coating (MnOx/Ti) and MnOx nanoparticles mixed with multi-walled carbon nanotubes (MnOx–CNTs/Ti) were fabricated by spraying–calcination method. The physicochemical properties of electrodes were investigated by SEM, XRD and XPS, which indicated that the surface coating of MnOx–CNTs/Ti, with MnOx nanoparticles dispersed uniformly on the CNTs, was smoother and with higher integrity than MnOx/Ti. Acid Red B was used as model pollutant to investigate the electro-catalytic activity of the electrodes, and the results revealed that the removal efficiency of Acid Red B reached 93.6% and 98.0% by MnOx/Ti and MnOx–CNTs/Ti, respectively, and the cell potential during the process of degradation by MnOx–CNTs/Ti was relatively low and stable. The electrochemical results confirmed that MnOx–CNTs/Ti possessed smaller charge transfer resistance and higher oxygen evolution current compared with MnOx/Ti, which can enhance the electro-catalytic activity and reduce the energy consumption by accelerating the transfer of electrons on the electrode surface. The accelerated lifetime tests of electrodes were carried out and showed that actual service lifetimes of MnOx–CNTs/Ti were 38 times of that for MnOx/Ti calculated by the experienced formula, which demonstrated that the durability of MnOx-based electrode was significantly promoted by addition of CNTs on Ti substrate.













Similar content being viewed by others
References
Kong Y, Wang Z, Wang Y et al (2011) Degradation of methyl orange in artificial wastewater through electrochemical oxidation using exfoliated graphite electrode. New Carbon Mater 26:459–464
Ureta-ZañArtu MS, Bustos P, Berríos C et al (2002) Electrooxidation of 2,4-dichlorophenol and other polychlorinated phenols at a glassy carbon electrode. Electrochim Acta 47:2399–2406
Zor S, Yazici B, Erbil M et al (1998) The electrochemical degradation of linearalkylbenzenesulfonate (LAS) on platinum electrode. Water Res 32:579–586
Iniesta J, Michaud PA, Panizza M et al (2001) Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim Acta 46:3573–3578
Manciu FS, Manciu M, Durrer WG et al (2014) A Drude model analysis of conductivity and free carriers in boron-doped diamond films and investigations of their internal stress and strain. J Mater Sci 49:5782–5789. https://doi.org/10.1007/s10853-014-8309-x
Feng YJ, Li XY (2003) Electro-catalytic oxidation of phenol on several metal-oxide electrodes in aqueous solution. Water Res 37:2399–2407
Duby P (1993) The history of progress in dimensionally stable anodes. JOM 45:41–43
Lin H, Niu J, Ding S et al (2012) Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2–Sb, Ti/SnO2–Sb/PbO2 and Ti/SnO2–Sb/MnO2 anodes. Water Res 46:2281–2289
Martínez-Huitle CA, De Battisti A, Ferro S et al (2008) Removal of the pesticide methamidophos from aqueous solutions by electrooxidation using Pb/PbO2, Ti/SnO2, and Si/BDD electrodes. Environ Sci Technol 42:6929–6935
Turkay O, Ersoy ZG, Barışçı S (2017) Review—the application of an electro-peroxone process in water and wastewater treatment. J Electrochem Soc 164:E94–E102
Kaur R, Kushwaha JP, Singh N (2018) Electro-oxidation of ofloxacin antibiotic by dimensionally stable Ti/RuO2 anode: evaluation and mechanistic approach. Chemosphere 193:685–694
Terezo A, Pereira EC (2002) Preparation and characterisation of Ti/RuO2 anodes obtained by sol–gel and conventional routes. Mater Lett 53:339–345
Zanta CLPS, de Andrade AR, Boodts JFC (1999) Solvent and support electrolyte effects on the catalytic activity of Ti/RuO2 and Ti/IrO2 electrodes: oxidation of isosafrole as a probe model. Electrochim Acta 43:3333–3340
Marshall AT, Haverkamp RG (2012) Nanoparticles of IrO2 or Sb–SnO2 increase the performance of iridium oxide DSA electrodes. J Mater Sci 47:1135–1141. https://doi.org/10.1007/s10853-011-5958-x
Massa A, Hernández S, Lamberti A et al (2017) Electro-oxidation of phenol over electrodeposited MnOx nanostructures and the role of a TiO2 nanotubes interlayer. Appl Catal B Environ 203:270–281
Nijjer S, Thonstad J, Haarberg GM (2001) Cyclic and linear voltammetry on Ti/IrO2–Ta2O5–MnOx electrodes in sulfuric acid containing Mn2+ ions. Electrochim Acta 46:3503–3508
Adams B, Tian M, Chen A (2009) Design and electrochemical study of SnO2-based mixed oxide electrodes. Electrochim Acta 54:1491–1498
Takashima T, Hashimoto K, Nakamura R (2012) Mechanisms of pH-dependent activity for water oxidation to molecular oxygen by MnO2 electrocatalysts. J Am Chem Soc 134:1519–1527
Zhang M, Gao J, Hong W et al (2019) Bimetallic Mn and Co encased within bamboo-like N-doped carbon nanotubes as efficient oxygen reduction reaction electrocatalysts. J Colloid Interface Sci 537:238–246
Ottone C, Armandi M, Hernández S et al (2015) Effect of surface area on the rate of photocatalytic water oxidation as promoted by different manganese oxides. Chem Eng J 278:36–45
Zhao K, Xu Z, He Z et al (2018) Vertically aligned MnO2 nanosheets coupled with carbon nanosheets derived from Mn-MOF nanosheets for supercapacitor electrodes. J Mater Sci 53:13111–13125. https://doi.org/10.1007/s10853-018-2562-3
Li P, Zhao YM, Ding BB et al (2015) Effect of calcination temperature and molar ratio of tin and manganese on capacitance of Ti/SnO2–Sb–Mn/β-PbO2 electrode during phenol electro-oxidation. J Electroanal Chem 747:45–52
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Fávero VO, Oliveira DA, Lutkenhaus JL et al (2018) Layer-by-layer nanostructured supercapacitor electrodes consisting of ZnO nanoparticles and multi-walled carbon nanotubes. J Mater Sci 53:6719–6728. https://doi.org/10.1007/s10853-018-2010-4
Duan XY, Ma F, Yuan ZX et al (2012) Comparative studies on the electro-catalytic oxidation performance of surfactant–carbon nanotube-modified PbO2 electrodes. J Electroanal Chem 677–680:90–100
Xing JT, Chen DH, Zhao XX et al (2015) Preparation and characterization of a novel porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode for the anodic oxidation of phenol wastewater. RSC Adv 5:5354–53513
Hu FP, Cui XW, Chen W et al (2010) Pulse electro-codeposition of Ti/SnO2–Sb2O4–CNT electrode for phenol oxidation. Electrochem Solid State Lett 13:F20–F23
Yang SM, Liang XP, Zhang D et al (2018) MnOx/Ti composite membrane anode in the electrocatalytic membrane reactor for phenolic wastewater treatment. J Electrochem Soc 165:E20–E27
Lee SW, Kim J, Chen S et al (2010) Carbon nanotube/manganese oxide ultrathin film electrodes for electrochemical capacitors. ACS Nano 4:3889–3896
Liu XW, Sun XF, Huang YX et al (2010) Nano-structured manganese oxide as a cathodic catalyst for enhanced oxygen reduction in a microbial fuel cell fed with a synthetic wastewater. Water Res 44:5298–5305
Reddy ALM, Shaijumon MM, Gowda SR et al (2009) Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano Lett 9:1002–1006
Correa-Lozano B, Comninellis C, Battisti AD (1997) Service life of Ti/SnO2–Sb2O5 anodes. J Appl Electrochem 27:970–974
Zhao GH, Cui X, Liu MC et al (2009) Electrochemical degradation of refractory pollutant using a novel microstructured TiO2 nanotubes/Sb-doped SnO2 electrode. Environ Sci Technol 43:1480–1486
Jiang HG, Ruhle M, Lavernia EJ (1999) On the applicability of the X-ray diffraction line profile analysis in extracting grain size and microstrain in nanocrystalline materials. J Mater Res 14:549–559
Dicastro V, Polzonetti G (1989) XPS study of MnO oxidation. J Electron Spectrosc 48:117–123
Wang Y, Cui JW, Luo L et al (2017) One-pot synthesis of NiO/Mn2O3 nanoflake arrays and their application in electrochemical biosensing. Appl Surf Sci 423:1182–1187
Dong H, Chen Y, Han M et al (2014) Synergistic effect of mesoporous Mn2O3-supported Pd nanoparticle catalysts for electrocatalytic oxygen reduction reaction with enhanced performance in alkaline medium. J Mater Chem A 2:1272–1276
Li Q, Liu JH, Zou JH et al (2011) Synthesis and electrochemical performance of multi-walled carbon nanotube/polyaniline/MnO2 ternary coaxial nanostructures for supercapacitors. J Power Sources 196:565–572
Hernández S, Ottone C, Varetti S et al (2016) Spin-coated vs. electrodeposited Mn oxide films as water oxidation catalysts. Materials 9:296
Takashima T, Hashimoto K, Nakamura R (2012) Inhibition of charge disproportionation of MnO2 electrocatalysts for efficient water oxidation under neutral conditions. J Am Chem Soc 134:18153–18156
Sokol Skii GV, Ivanova SV, Ivanova ND et al (2012) Doped manganese (IV) oxide in processes of destruction and removal of organic compounds from aqueous solutions. J Water Chem Technol 34:227–233
Peng WC, Wang SB, Li XY (2016) Shape-controlled synthesis of one-dimensional α-MnO2 nanocrystals for organic detection and pollutant degradation. Sep Purif Technol 163:15–22
Xu L, Song XL (2015) A novel Ti/antimony-doped tin oxide nanoparticles electrode prepared by screen printing method and its application in electrochemical degradation of C.I. Acid Red 73. Electrochim Acta 185:6–16
Duan XY, Zhao YY, Liu W et al (2014) Electrochemical degradation of p-nitrophenol on carbon nanotube and Ce-modified-PbO2 electrode. J Taiwan Inst Chem E 45:2975–2985
Guo CY, Li H, Zhang X et al (2015) 3D porous CNT/MnO2 composite electrode for high-performance enzymeless glucose detection and supercapacitor application. Sens Actuators B Chem 206:407–414
Montilla F, Morallón E, De Battisti A et al (2004) Preparation and characterization of antimony-doped tin dioxide electrodes. Part 1. Electrochemical characterization. J Phys Chem B 108:5036–5043
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants. Electrochim Acta 39:1857–1862
Liu A, Liu K, Zhou H et al (2018) Solution evaporation processed high quality perovskite films. Sci Bull 63:1591–1596
Chen K, Li Wei HJ, Xu Y et al (2019) Untying thioether bond structure enabled by “Voltage-Scissors” for stable room temperature sodium-sulfur batteries. Nanoscale 11:5967–5973
Guo J, Xu Y, Wang C (2011) Sulfur-impregnated disordered carbon nanotubes cathode for lithium–sulfur batteries. Nano Lett 11:4288–4294
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant No. 21503217) and Fundamental Research Funds for the Central Universities (Nos. 3132016059, 3132016326).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, X., Zhao, J., Zhou, Z. et al. Effect of multi-walled carbon nanotubes addition on MnOx/Ti electrode prepared by spraying–calcination method for electro-catalytic oxidation of Acid Red B. J Mater Sci 54, 12509–12521 (2019). https://doi.org/10.1007/s10853-019-03731-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03731-1