Abstract
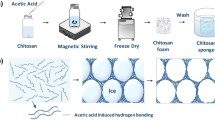
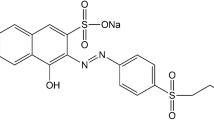
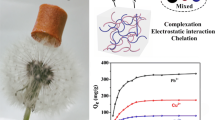
Effective wastewater remediation requires the development of suitable adsorbents. In this study, a strategy based on electrostatic interactions between the amino groups of chitosan and the anionic groups of acid dyes was developed for the removal of acid dyes from aqueous solution. By adjusting the fabrication parameters, chitosan/alginate porous sponges with controlled specific areas and pore structures were obtained. To enlarge the adsorption area, the adsorbents were processed via a freezing and lyophilization method to form three-dimensional porous sponges. Acid dyes in aqueous solution were adsorbed by the porous sponges, mainly through an electrostatic interaction mechanism. When the molar ratio of chitosan to alginate was fixed at 1.5:1, the porous sponges showed a maximum adsorption removal capacity of 1468.9 ± 16.3 mg g−1 for acid red B14 at a dye concentration of 150 mg L−1 and pH of 2. The adsorption capacity of this system was significantly enhanced compared with those of control groups such as chitosan powder. Thus, chitosan porous scaffolds provide a novel method for improving the removal capacities of chitosan-based materials for cleanup of acid–dye-contaminated water.







Similar content being viewed by others
References
Ratnamala G, Brajesh K (2013) Biosorption of remazol navy blue dye from an aqueous solution using pseudomonas putida. Int J Environ Sci Te 2(1):80–89
Attia AA, Rashwan WE, KhedrS SA (2006) Capacity of activated carbon in the removal of acid dyes subsequent to its thermal treatment. Dyes Pigments 69:128–136
Wang JH, Zhang Z, Zhang Q, Liu JH, Ma JQ (2018) Preparation and adsorption application of carbon nanofibers with large specific surface area. J Mater Sci 53:16466–16475. https://doi.org/10.1007/s10853-018-2772-8
Rêgo T, Cadaval T Jr, Dotto G, Pinto L (2013) Statistical optimization, interaction analysis and desorption studies for the azo dyes adsorption onto chitosan films. J Colloid Interfaces Sci 411:27–33
Xing JS, Wang XJ, Xun JJ, Peng J, Xu Q, Zhang WX, Lou T (2018) Preparation of micro-nanofibrous chitosan sponges with ternary solvents for dye adsorption. Carbohyd Polym 198:69–75
Vakili M, Rafatullah M, Salamatiniab B et al (2014) Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohyd Polym 113:115–130
Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K (2017) Chitosan as a bioactive polymer: processing, properties and applications. Int J Biol Macromol 105:1358–1368
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan—a versatile semisynthetic polymer in biomedical applications. Prog Polym Sci 36:981–1014
Esquerdo VM, Cadaval TRS Jr, Dotto GL, Pinto LAA (2014) Chitosan scaffold as an alternative adsorbent for the removal of hazardous food dyes from aqueous solutions. J Colloid Interfaces Sci 424:7–15
Bekc IZ, Özveri C, Seki Y, Yurdakoc K (2008) Sorption of malachite green on chitosan bead. J Hazard Mater 154(1):254–261
Martinez L, Agnely F, Leclerc B, Siepmann J, Cotte M, Deiger S, Couarraze G (2007) Cross-linking of chitosan and chitosan poly(ethylene oxide) beads: a theoretical treatment. Eur J Pharm Biopharm 67:339–348
Li CG, Wang F, Peng WG, He YH (2013) Preparation of chitosan and epichlorohydrin cross-linked adsorbents and adsorption property of dyes. Appl Mech Mater 423:584–587
Chan MY, Husseinsyah S, Sam ST (2013) Chitosan/corn cob biocomposite films by cross-linking with glutaraldehyde. BioResources 8(2):2910–2923
Grégorio C (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30:38–70
Guibal E (2005) Heterogeneous catalysis on chitosan-based materials: a review. Prog Polym Sci 30:71–109
Azlan K, Wansaime WN, Lai Ken L (2009) Chitosan and chemically modified chitosan beads for acid dyes sorption. J Environ Sci 21(3):296–302
Chatterjee S, Chatterjee T, Lim SR, Woo SH (2011) Effect of surfactant impregnation into chitosan hydrogel beads formed by sodium dodecyl sulfate gelation for the removal of congo red. Sep Sci Technol 46(13):2022–2031
Li CY, Lou T, Yan X, Long YZ, Cui GP, Wang XJ (2018) Fabrication of pure chitosan nanofibrous membranes as effective absorbent for dye removal. Int J Biol Macromol 106:768–774
Martin RV, Brenda VR, Rodrigo RZ, Daniel ASK, Luis FQO (2015) Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed Res Int 2015:821279
Lu GY, Zhu L, Kong LJ, Zhang L, Gong YD, Zhao NM (2006) Porous chitosan MCs for large scale cultivation of cells for tissue engineering: fabrication and evaluation. Tsinghua Sci Technol 11:427–432
Fang JJ, ZhangY Yan SF, Liu ZW, He SM, Cui L, Yin JB (2014) Poly(l-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater 10:276–288
Cui Z, Liu C, Lu T, Xing W (2007) Polyelectrolyte complexes of chitosan and phosphotungstic acid as proton-conducting membranes for direct methanol fuel cells. J Power Sources 167:94–99
Holder KM, Smith RJ, Grunlan JC (2017) A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J Mater Sci 52:12923–12959. https://doi.org/10.1007/S10853-017-1390-1
Ilina AV, Varlamov VP (2005) Chitosan-based polyelectrolyte complexes: a review. Appl Biochem Microbiol 41:5–11
Yan SF, Zhang KX, Liu ZW, Xin Zhang, Gan Lu, Cao B, Chen XS (2013) Fabrication of poly(l-glutamic acid)/chitosan polyelectrolyte complex porous scaffolds for tissue engineering. J Mater Chem B 1:1541–1551
Hamano T, Chiba D, Teramoto A, Kondo Y, Abe K (1998) Effect of polyelectrolyte complex (PEC) on human periodontal ligament Fibroblast (HPLF) functions in the presence of glucocorticoids. J Biomater Sci Polym Ed 9:985–1000
Zhou ZH, Cao DF, Liu LH et al (2013) Fabrication and properties of gelatin/chitosan microspheres loaded with 5-fluorouracil. J Macromol Sci B 52:973–983
Zhou ZH, Liu LH, Liu QQ et al (2012) Study on controlled release of 5-fluorouracil from gelatin/chitosan microspheres. J. Macromol Sci A 49:1030–1034
Reed S, Wu BM (2017) Biological and mechanical characterization of chitosan-alginate scaffolds for growth factor delivery and chondrogenesis. J Biomed Mater Res B Appl Biomater 105(2):272–282
Sneha K (2017) Self-functionalized, oppositely charged chitosan-alginate scaffolds for biomedical applications. Biotechnol Ind J 13(2):1–15
Florczyk SJ, Kievit FM, Wang K, Erickson AE, Ellenbogen RG, Zhang MQ (2016) 3D porous chitosan-alginate scaffolds promote proliferation and enrichment of cancer stem-like cells. J Mater Chem B Mater Biol Med 4(38):6326–6334
Zhou ZH, Zhou JN, Liu LH, Yi QF, Liu QQ, Zeng WN, Yang ZM (2012) Fabrication and characterization of gelatin/chitosan microspheres for drug release. J Macromol Sci B 51:777–785
Zeng S, Cui ZX, Yang ZQ et al (2016) Characterization of highly interconnected porous poly(lactic acid) and chitosan-coated poly(lactic acid) scaffold fabricated by vacuum-assisted resin transfer molding and particle leaching. J Mater Sci 51:9958–9970. https://doi.org/10.1007/s10853-016-0203-2
He JX, Tan WL, Han QM, Cui SZh, Shao WL, Sang F (2016) Fabrication of silk fibroin/cellulose whiskers–chitosan composite porous scaffolds by layer-by-layer assembly for application in bone tissue engineering. J Mater Sci 51:4399–4410. https://doi.org/10.1007/s10853-016-9752-7
Shimizu Y, Taga A, Yamaoka H (2003) Synthesis of novel crosslinked chitosans with a higher fatty diacid diglycidyl and their adsorption abilities towards acid dyes. Adsorpt Sci Technol 21(5):439–449
Azlan K, Wansaime WN, Lai Ken L (2009) Chitosan and chemically modified chitosan beads for acid dyes sorption. J Environ Sci-China 21(3):296–302
Ong ST, Seou CK (2013) Removal of reactive black 5 from aqueous solution using chitosan beads: optimization by Plackett–Burman design and response surface analysis. Desalin Water Treat 52:7673–7684
Phung YP, Ong ST, Keng PS (2013) Determination of important parameters in affecting the uptake of reactive black 5 by chitosan beads through statistical approach. J Chemny 2013:1–7
Sugimoto M, Morimoto M, Sashiwa H, Saimoto H, Shigemasa Y (1998) Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohydr Polym 36(1):49–59
Sankalia MG, Mashru RC, Sankalia JM, Sutariya VB (2007) Reversed chitosan–alginate polyelectrolyte complex for stability improvement of alpha-amylase: optimization and physicochemical characterization. Eur J Pharm Biopharm 65:215–232
Gümüsoglu T, Arı GA, Deligöz H (2011) Investigation of salt addition and acid treatment effects on the transport properties of ionically cross-linked polyelectrolyte complex membranes based on chitosan and polyacrylic acid. J Membr Sci 376:25–34
Crini G, Gimbert F, Robert C et al (2008) The removal of basic blue 3 from aqueous solutions by chitosan-based adsorbent: batch studies. J Hazard Mater 153(1–2):96–106
Kyzas GZ, Kostoglou M, Vassiliou AA, Lazaridis NK (2011) Treatment of real effluents from dyeing reactor: experimental and modeling approach by adsorption onto chitosan. Chem Eng J 168(2):577–585
Tajizadegan H, Torabi O, Heidary A, Golabgir MH, Jamshidi A (2015) Study of methyl orange adsorption properties on ZnO–Al2O3 nanocomposite adsorbent particles. Desalinat Water Treat 57:12324–12334
Xin S, Zeng Z, Zhou X et al (2017) Recyclable Saccharomyces cerevisiae loaded nanofibrous mats with sandwich structure constructing via bio-electrospraying for heavy metal removal. J Hazard Mater 324:365–372
Madihally SV, Matthew HW (1999) Porous chitosan scaffolds for tissue engineering. Biomaterials 20:1133–1142
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant nos. 81701844, 51773057 and 81701837), the Natural Science Foundation of Fujian Province (2017J05029) and Department of Education of Fujian Province (JAT160343), the National Experimental Teaching Demonstration Center of Chemical Engineering and Materials (2016 [7]), IUC Program of Hunan Provincial Education Department (No. 15CY004) and the Open-end Funds of Fujian Engineering, and Research Center of Rural Sewage Treatment and Water Safety (EBL2018006). We would also like to thank Editage [www.editage.cn] for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, M., Wu, W., Fang, J. et al. Fabrication of chitosan/alginate porous sponges as adsorbents for the removal of acid dyes from aqueous solution. J Mater Sci 54, 9995–10008 (2019). https://doi.org/10.1007/s10853-019-03602-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03602-9