Abstract
Sodium aluminum hydride has been extensively investigated for hydrogen storage applications whereas its intermediate decomposition compound Na3AlH6 received much less attention, despite having a lower dissociation pressure and a reasonable hydrogen storage capacity of 3.0 wt%. In this work, Na3AlH6 is synthesized through ball milling, starting from NaAlH4 and 2 NaH in the presence of TiCl3 catalyst precursor, and evaluated on its hydrogen sorption properties and cycle stability. Further addition of 8 mol% Al and 8 mol% activated carbon (AC) and their effect on both the hydrogen sorption properties and cycle stability have been investigated. In order to explore whether the introduction of the Al and AC additives would be more beneficial (in terms of hydrogen sorption behavior and cycle stability) after the Na3AlH6 synthesis or during its synthesis, pre-synthesized Na3AlH6-based measurements were also included in this work. TiCl3-catalyzed NaAlH4 + 2 NaH sample showed a stable reversible hydrogen storage capacity of 1.7 wt%, which was further increased to 2.1 wt% with the addition of Al-powder and activated carbon AC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Complex metal hydrides have gained much interest as hydrogen storage materials for practical applications due to their relative high theoretical gravimetric hydrogen capacity. Unfortunately, many complex metal hydrides require high pressures and temperatures for their hydrogenation and high temperature for dehydrogenation. Researchers have therefore focused on altering the thermodynamics for dehydrogenation of these complex metal hydrides to lower the temperature of hydrogen release and improving the kinetics for the hydrogenation and dehydrogenation reactions [1, 2]. Among the complex metal hydrides, sodium alanate, NaAlH4, has acquired much attention as a potential hydrogen storage material since it was reported to be reversible through doping with small amounts of Ti-catalysts [3]. NaAlH4 has a theoretical reversible hydrogen storage capacity of 5.6 wt% and releases its hydrogen according to Eqs. (1) and (2).
Despite its lower hydrogen capacity of 3.0 wt%, Na3AlH6 has attracted also attention as a potential hydrogen storage material, because of much lower dissociation pressure than NaAlH4 (6 vs. 66 bar at 150 °C, respectively) and therefore making it more suitable for practical applications [3]. Formed in the first decomposition step of NaAlH4 (1), Na3AlH6 can also be directly synthesized through the reaction of 2 NaH and NaAlH4 in heptane at 160 °C [4] or through hydrogenation of Na and Al in toluene at 165 °C [5]. More recently, direct synthesis of β-Na3AlH6 was also achieved through mechanical milling of NaAlH4 and 2 NaH [6] and from NaH and Al in the presence of 4 mol% TiF3 under hydrogen pressure [7]. Furthermore, it was found that Na3AlH6 synthesized through mechanical milling exhibited faster kinetics than Na3AlH6 obtained from the decomposition of NaAlH4 [8]. Na3AlH6 proved to be reversible through the addition of Ti compounds [3]; however, doping with Zr compounds was reported to have better effect on the dehydrogenation of Na3AlH6 than doping with Ti [8, 9]. Although a large number of experimental results have been described on NaAlH4, a small number of studies have been reported on Na3AlH6 and its use as a hydrogen storage material. So far the studies on Na3AlH6 deal with theoretical calculations of structural parameters, effect of Ti doping, and hydrogen diffusion in pure and Ti-doped Na3AlH6. To our knowledge, no extensive study on the direct synthesis of Na3AlH6 through mechanochemical processes and the effect of various additives on the hydrogenation and dehydrogenation behavior has been reported so far. In this work, we investigate the synthesis of Na3AlH6 starting from NaAlH4 and NaH in the presence of TiCl3, combined with the addition of Al and AC. It has been reported that addition of AC to Ti-doped NaAlH4 not only resulted in improved dehydrogenation and hydrogenation kinetics but also enhanced the cycle life [10] and ability to conduct heat [11]. We also investigate whether the additives are best added during or after the Na3AlH6 synthesis in order to obtain the optimum performance of the material. The long-term cycle stability measurements are performed at 170 °C, which allows using this material in combination with HT-PEM fuel cells.
Experimental
The starting materials NaH (60% dispersion in mineral oil, Sigma-Aldrich Chemical) and NaAlH4 (technical grade, Sigma-Aldrich Chemical) were further purified to obtain 98% purity. NaH was cleaned from mineral oil by washing with pentane and NaAlH4 was purified by dissolving in THF solution, and then recrystallized using toluene. TiCl3 (ReagentPlus®, Sigma-Aldrich Chemical), AC (activated carbon) (0.79 cm3 g−1, Carbo Tech), Al (99%, Aluminum Rheinfelden), and doped NaAlH4 with 4 mol% TiCl3 (prepared in house) were used without further purification. The samples (2.2 g) were prepared by mechanical milling for 4 h under inert atmosphere (Ar) using hardened steel grinding bowls and grinding balls (ø = 10 mm) in a Fritsch Planetary Mono Mill Pulverisette 6 at 450 rpm, with a ball-to-powder ratio of 30:1.
The samples characterized by X-ray diffraction were measured in transmission mode with a curved germanium (111) monochromator (STOE STADI P). Five samples: as-received NaH, as-received NaAlH4, as-milled NaAlH4 + 4 mol% TiCl3, NaAlH4 + 4 mol% TiCl3 + 2 NaH, and NaAlH4 + 4 mol% TiCl3 + 2 NaH + 8 mol% Al + 8 mol% AC were characterized using an image plate detector, Cu-Kα radiation (1.541 Å), and capillary (ø = 0.5 mm). Three as-milled samples: Na3AlH6, Na3AlH6 + 4 mol% TiCl3, and Na3AlH6 + 4 mol% TiCl3 + 8 mol% Al + 8 mol% AC were characterized using a Mythen1K detector, Mo-radiation (0.7093 Å), and capillary (ø = 0.5 mm).
Differential scanning calorimetry (DSC) measurements were carried out with a Mettler-Toledo TGA/DSC1 Stare System, under 1 bar of flowing argon (50 ml min−1) with a heating rate of 10 °C min−1 from room temperature to a maximum temperature of 450 °C. Thermal gravimetric analysis (TGA) measurements were carried out with a NETZSCH STA 449 C Jupiter, under 1 bar of flowing argon (50 ml min−1) with a heating rate of 10 °C min−1 (Na3AlH6-based samples) or 2 °C min−1 (NaAlH4-based samples) from room temperature to a maximum temperature of 450 °C. Handling and storing of samples were performed in an inert atmosphere (Ar) glove box (MBraun Unistar) with < 1 ppm O2 and < 1 ppm H2O.
Absorption–desorption cycle measurements were performed on a PCT-Pro 2000 (SETARAM). The tests were carried out using 2 g of material which was placed in the sample holder using an argon-filled glove box. Several stainless steel spacers were used to center the sample inside the sample holder, thereby reducing the empty space of the sample holder and increasing the accuracy of the measurement. The pressure was monitored using an MKS pressure transducer with an accuracy of 1.0% of the reading value. The temperature of the sample was measured inside the sample holder well using a type K thermocouple with an accuracy of ± 1.1 °C. The mass of the samples was measured on SCALTEC SBA 42 balance with an accuracy of ± 0.003 g. The cycling conditions were performed at 170 °C, with 2-h hydrogenation cycles at 25 bar hydrogen pressure and 2-h dehydrogenation cycles at 0.1 bar hydrogen pressure.
Results and discussion
Ball-milled samples
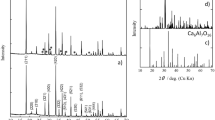
Figure 1 shows the X-ray diffraction (XRD) patterns of the starting materials NaH (Fig. 1a) and NaAlH4 (Fig. 1b) together with as-milled NaAlH4 containing samples. It was observed that ball milling (4 h) of NaAlH4 in the presence of 4 mol% TiCl3 resulted in the partial decomposition of NaAlH4 as diffraction peaks of Na3AlH6, NaCl, and Al were found (Fig. 1c). Synthesis of Na3AlH6 was best achieved through ball milling of 2 mol of NaH and NaAlH4, in the presence of 8 mol% of Al and AC (Fig. 1e) which showed (in contrary to Fig. 1d) no diffraction peaks of remaining NaH. Although TiCl3 has been successfully applied as a dopant for sodium alanate systems, the catalytic mechanism remains unclear. It has been reported that during ball milling the titanium of TiCl3 is reduced to its metallic state [12]. However, addition of metallic titanium to NaAlH4 resulted in lower dehydrogenation capacities, indicating that the reduction in the dopant plays more complex role than presumed. The same applies for the Al–Ti compounds that have been proposed as the active species (rather than Ti), but when directly added, the similar results as for non-doped samples were obtained. Bogdanović et al. and Singh et al. reported that although the formation of NaCl as a by-product reduces the overall reversible hydrogen capacity, it acts as a grain refiner for NaH. Similarly, AlxTiy species have been found to act as a grain refiner for Al as well, preventing particle growth [12,13,14]. The presence of grain refiners in combination with the vacancies and defects created during the ball-milling process seems to be crucial. It improves the mass transfer during hydrogenation and dehydrogenation, allowing faster kinetics. The absence of grain refiners would explain the poor results obtained for systems in which metallic Ti or Al–Ti was directly added [14].
In order to investigate the doping effect of the various additives, pre-synthesized Na3AlH6 was used (Fig. 2). XRD measurements showed that addition of 4 mol% TiCl3 alone did not result in a significant decomposition of Na3AlH6 (Fig. 2b), the weak NaAlH4 diffraction peak at 2θ values of 22° was already present in the pre-synthesized Na3AlH6 (Fig. 2a). Furthermore, addition of 8 mol% of both Al and AC showed no appearance of new phases (Fig. 2c). In fact, samples (b) and (c) are very similar.
Sandrock et al. [15] reported that although TiCl3 enhances the dehydriding and hydriding kinetics for both NaAlH4 and Na3AlH6, the effect is more pronounced for NaAlH4 than for Na3AlH6 (activation energy (desorption) for non-catalyzed NaAlH4 = 118 kJ mol−1 (H2), catalyzed (4 mol% TiCl3) NaAlH4 = 80 kJ mol−1 (H2), non-catalyzed Na3AlH6 = 121 kJ mol−1 (H2), catalyzed (4 mol% TiCl3) Na3AlH6 = 98 kJ mol−1 (H2)). From the obtained XRD data, it seems that Na3AlH6 is less reactive toward the TiCl3 catalyst than NaAlH4, as no partial decomposition of Na3AlH6 was observed (i.e., formation of NaCl and NaH). This was further emphasized by in situ pressure recording measurements during ball milling (Fig. 3) which showed that the pressure (from released hydrogen during the reduction of TiCl3) inside the milling vial increased to 5.2 bar for catalyzed NaAlH4, but only to 0.7 bar for catalyzed Na3AlH6. These results allowed for rough estimation of the molar quantity of NaCl produced assuming all TiCl3 has reacted, giving overall Eq. (3) and reversible hydrogen storage of 2.46 wt% for NaAlH4 + 2 NaH + 4 mol% TiCl3 as shown in Eq. (4). For the additive containing sample, the Al addition results in a slightly higher reversible hydrogen capacity (2.61 wt%), see Eqs. (5) and (6).
Thermal dehydrogenation
Thermal decomposition behavior of the NaAlH4-based samples was investigated using DSC measurements. Figure 4 shows the DSC curves of as-received starting materials NaH (Fig. 4a) and NaAlH4 (Fig. 4b) together with milled samples when heated from room temperature to 450 °C under 1 bar of flowing argon at a heating rate of 10 °C min−1. NaAlH4 in the presence of the TiCl3 catalyst precursor (Fig. 4c) showed small and weak endothermic events at 139 and 396 °C, corresponding to the decomposition of NaAlH4 and NaH which both occurred at much lower temperatures than their respective as-received materials, demonstrating the kinetic effect of the catalyst precursor. The weak and broad endothermic event between 169 and 227 °C is contributed to the decomposition of Na3AlH6 (supported by TGA data, Fig. 7), whereas the cause for the exothermic event at 243 °C remains unclear. The most pronounced effect was observed for the sample containing ball-milled NaAlH4 in the presence of 2 mol of NaH and 4 mol% TiCl3 (Fig. 4d), which showed a double endothermic peak for the decomposition of Na3AlH6 between 225 and 250 °C, followed by the endothermic peak for the decomposition of NaH around 390 °C. No endothermic events corresponding to NaAlH4 were observed, further supporting the full conversion of NaAlH4 into Na3AlH6 during the ball-milling process as observed from XRD data (Fig. 1d). Further addition of Al and AC (Fig. 4e) resulted in a similar curve as obtained for the TiCl3-doped sample (Fig. 4c).
DSC curves for a as-received NaH, b as-received NaAlH4, c milled NaAlH4 + 4 mol% TiCl3, d milled NaAlH4 + 2 NaH + 4 mol% TiCl3, and e milled NaAlH4 + 2 NaH + 4 mol% TiCl3 + 8 mol% Al + 8 mol% AC. Samples were heated to 450 °C under 1 bar of flowing argon at 10 °C min−1. *, melting of NaAlH4; •, decomposition of NaAlH4; ♠, decomposition of Na3AlH6; ∆, decomposition of NaH
Figure 5 shows the DSC measurements for the Na3AlH6 containing milled samples which displayed no significant change for the thermal events, as found for the NaAlH4 containing samples, when heated from room temperature to 450 °C under 1 bar of flowing argon at a heating rate of 10 °C min−1. As-received Na3AlH6 showed two main endothermic events, the first is a complex event between 243 and 304 °C, corresponding to the decomposition of Na3AlH6 into elemental Al and NaH. The second endothermic signal which occurred at 385 °C was caused by the decomposition of NaH. For the TiCl3-catalyzed Na3AlH6 sample (Fig. 5b), the endothermic signal for the Na3AlH6 decomposition step was observed at much lower temperatures (185–278 °C), whereas the endothermic peak for the decomposition of NaH took place at a higher temperature (391 °C). However, the lowest peak temperatures were obtained for the catalyzed Na3AlH6 sample with additional 8 mol% of Al and AC (Fig. 5c) with the Na3AlH6 decomposition reaction between 177 and 265 °C and the NaH decomposition peak at 370 °C.
Na3AlH6 is known to undergo phase transformation of α-Na3AlH6 to β-Na3AlH6 around 252 °C [15, 16], and in order to detect this phase transformation rapid heating and fast data acquisition is needed [17]. Weidenthaler et al. [18] investigated the phase transformation using in situ DSC and high-temperature X-ray diffraction methods and found that using different heating rate resulted in different decomposition behavior. For example, they observed that the endothermic signals for the decomposition of Na3AlH6 and NaH shifted to higher temperatures with higher heating rates. A shoulder (~ 248 °C) at the Na3AlH6 decomposition signal became apparent at heating rates > 10 K min−1, suggesting that another reaction is taken place prior to the decomposition of Na3AlH6 [18]. Figure 5a shows a shoulder at 256 °C which is believed to be the phase transformation of α-Na3AlH6 to β-Na3AlH6. However, in the doped samples (Fig. 5b, c) this shoulder is not observed and is most likely due to increased kinetics resulting in the decomposition of α-Na3AlH6 to NaH and Al without detectable formation of β-Na3AlH6.
The dehydrogenation curves for the NaAlH4- and Na3AlH6-based samples are shown in Figs. 6 and 7, respectively, with their corresponding hydrogen release capacities listed in Table 1. As-received NaAlH4 (Fig. 6a) showed an onset dehydrogenation temperature of 189 °C and hydrogen release capacity of 5.5 wt%, which is close to its theoretical capacity of 5.6 wt%. However, addition of 4 mol% of TiCl3 (Fig. 6b) resulted in an onset dehydrogenation temperature of 90 °C, a shift of roughly 100 °C compared to the pristine material, releasing hydrogen capacity of 4.2 wt%. For Na3AlH6, the effect of TiCl3 was less pronounced (Fig. 7b) and resulted in a shift of roughly 50 °C to lower temperatures and hydrogen release capacity of 2.3 wt% compared to 2.4 wt% for the pristine Na3AlH6 (Table 1). From XRD data (Fig. 2b), this small effect was expected as no change was observed compared to pristine Na3AlH6.
For the sample containing both 4 mol% TiCl3 and 2 mol NaH (Fig. 6c), the onset dehydrogenation temperature shifted to higher temperatures in comparison with the sample containing 4 mol% TiCl3 (Fig. 6b) and the sample containing 4 mol% TiCl3, 2 mol NaH, 8 mol% Al, and 8 mol% AC (Fig. 6d) and occurred at 206 °C, providing a hydrogen release capacity of 2.0 wt% (Table 1). From XRD data (Fig. 1d), it could be observed that Na3AlH6 which was synthesized in situ through the addition of NaH resulted in a lower onset dehydrogenation temperature than found for as-received Na3AlH6 (233 °C), Fig. 7a. In Fig. 6d, it can be seen that the hydrogen release capacity increased to 2.5 wt% (Table 1) when 8 mol% of Al and AC were added to the NaAlH4 + 2 NaH + 4 mol% TiCl3 sample and gave an onset dehydrogenation temperature of 149 °C. Similar results were obtained for the doped Na3AlH6 sample when 8 mol% of both Al and AC were added, showing an onset dehydrogenation temperature of 171 °C and a hydrogen release capacity of 2.6 wt%. It seems that, for both the NaAlH4- and Na3AlH6-based samples, the Al and AC additives reduced the onset dehydrogenation temperatures further, possibly by increasing the dehydrogenation kinetics and the increased hydrogen release capacity through addition of Al as proposed in Eqs. (5) and (6).
Cycle stability measurements
Figure 8 shows the cycle stability measurement for the Na3AlH6 + 4 mol% TiCl3 sample when subjected to 2-h hydrogenation (28 bar H2)/dehydrogenation (0.1 bar H2) cycles at 170 °C. The sample showed good cycle stability with a hydrogen capacity of 1.6 wt% at the start of the measurement which increased slightly to 1.7 wt% after 750 h [19]. No capacity loss was observed till 650 h of operation. However, at 650 h the capacity loss of ~ 10–15% which lasted only for a few cycles (Fig. 8) was observed. This capacity loss was not observed for the Na3AlH6 + 4 mol% TiCl3 + 8 mol% Al + 8 mol% AC sample and it’s unclear as to the reason for its occurrence.
Addition of 8 mol% Al and 8 mol% AC to the Na3AlH6 with 4 mol% TiCl3 (same cycling conditions as for the Na3AlH6 + 4 mol% TiCl3 sample) resulted in an increased hydrogen capacity of 2.2 wt% (Fig. 9) in comparison with 1.7 wt% (Fig. 8). The sample showed good cycle stability and in contrary to the Na3AlH6 + 4 mol% TiCl3 sample, no capacity losses were observed during 750 h of operating. Electricity shutdown caused some interruption at around 50, 170, 250, and 480 h since beginning of the experiment.
Conclusions
Ball-milled sample containing NaAlH4 + 2 NaH + 4 mol% TiCl3 + 8 mol% Al + 8 mol% AC gave better results compared to the sample containing NaAlH4 + 2 NaH + 4 mol% TiCl3, with hydrogen capacities of 2.2 and 1.7 wt%, respectively. The addition of Al allowed for the remaining NaH to react further into Na3AlH6 during the hydrogenation process, thereby increasing the hydrogen capacity. Cycle life stability measurements for both samples showed no sign of performance loss up to 750 h of operation, allowing the use as hydrogen storage materials in combination with HT-PEM fuel cell applications.
References
Jain IP, Jain P, Jain A (2010) Novel hydrogen storage materials: a review of lightweight complex hydrides. J Alloys Compd 503:303–339
Sakintuna B, Lamari-Darkrim F, Hirscher M (2007) Metal hydride materials for solid hydrogen storage: a review. Int J Hydrogen Energy 32:1121–1140
Bogdanović B, Schwickardi M (1997) Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J Alloys Compd 253–254:1–9
Zakharkin LI, Gavrilenko VV (1962) The direct synthesis of sodium and potassium aluminum hydrides from the elements. Dokl Akad Nauk SSSR 145:793–796
Ashby EC, Kobetz P (1966) The direct synthesis of Na3AlH6. Inorg Chem 5:1615–1617
Huot J, Boily S, Guther V, Schulz R (1999) Synthesis of Na3AlH6 and Na2LiAlH6 by mechanical alloying. J Alloys Compd 283:304–306
Wang P, Kang XD, Cheng HM (2005) Direct formation of Na3AlH6 by mechanical milling NaH/Al with TiF3. Appl Phys Lett 87:071911
Kircher O, Fichtner M (2005) Kinetic studies of the decomposition of NaAlH4 doped with a Ti-based catalyst. J Alloys Compd 404–406:339–342
Zidan RA, Takara S, Hee AG, Jensen CM (1999) Hydrogen cycling behavior of zirconium and titanium–zirconium-doped sodium aluminum hydride. J Alloys Compd 285:119–122
Mao J, Guo Z, Liu H (2012) Enhanced hydrogen storage properties of NaAlH4 co-catalysed with niobium fluoride and single-walled carbon nanotubes. RSC Adv 2:1569–1576
Adelhelm P, de Jongh PE (2011) The impact of carbon materials on the hydrogen storage properties of light metal hydrides. J Mater Chem 21:2417–2427
Léon A, Schild D, Fichtner M (2005) Chemical state of Ti in sodium alanate doped with TiCl3 using X-ray photoelectron spectroscopy. J Alloys Compd 404–406:766–770
Bogdanović B, Eberle U, Felderhoff M, Schüth F (2007) Complex aluminium hydrides. Scr Mater 56:813–816
Singh S, Eijt SWH, Huot J, Kockelmann WA, Wagemaker M, Mulder FM (2007) The TiCl3 catalyst in NaAlH4 for hydrogen storage induces grain refinement and impacts on hydrogen vacancy formation. Acta Mater 55:5549–5557
Sandrock G, Gross K, Thomas G (2002) Effect of Ti-catalyst content on the reversible hydrogen storage properties of the sodium alanates. J Alloys Compd 339:299–308
Bastide J-P, Bonnetot B, Létoffé J-M, Claudy P (1981) Polymorphisme de l’hexahydroaluminate trisodique Na3AlH6. Mater Res Bull 16:91–96
Bogdanović B, Brand RA, Marjanović A, Schwickardi M, Tölle J (2000) Metal-doped sodium aluminium hydrides as potential new hydrogen storage materials. J Alloys Compd 302:36–58
Weidenthaler C, Pommerin A, Felderhoff M, Schmidt W, Bogdanović B, Schüth F (2005) Evidence for the existence of β-Na3AlH6: monitoring the phase transformation from α-Na3AlH6 by in situ methods. J Alloys Compd 398:228–234
Urbancyk R, Peinecke K, Felderhoff M, Hauschild K, Kersten W, Peil S, Bathen D (2014) Aluminium alloy based hydrogen storage tank operated with sodium aluminium hexahydride Na3AlH6. Int J Hydrogen Energy 39:17118–17128
Acknowledgements
Open access funding provided by Max Planck Society. Financial support by Ministerium für Wirtschaft, Energie, Bauen, Wohnen und Verkehr des Landes Nordrhein-Westfalen and European Regional Development Fund (ERDF) (Funding No. ef003a) is much appreciated. The authors gratefully acknowledge the Max Planck Society for basic funding. The authors thank Dr. Claudia Weidenthaler and Jan Ternieden for measurement and interpretation of X-ray diffraction patterns.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Peinecke, K., Meggouh, M. & Felderhoff, M. Mechanochemical synthesis and effect of various additives on the hydrogen absorption–desorption behavior of Na3AlH6. J Mater Sci 53, 13742–13750 (2018). https://doi.org/10.1007/s10853-018-2279-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2279-3