Abstract
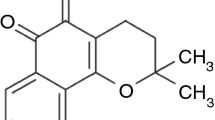
Benznidazole (BNZ) is the main drug used for the treatment of Chagas disease. However, its low aqueous solubility limits its efficacy. In this study, we have prepared host–guest inclusion complexes of BNZ with beta-cyclodextrin (β-CD) in solid phase and assessed the influence of the co-solvents triethanolamine (TEA) and 1-methyl-2-pyrrolidone (NMP) for improving the drug dissolution performance. The physicochemical properties of prepared spray-dried (SD) particles and physical mixtures (PM) were carefully monitored using FTIR, XRD and SEM images. The used co-solvents affected β-CD–drug interactions, the structural properties and drug dissolution behavior of particles. TEA did not improve the solubilizing effect of β-CD, while NMP was able to enhance drug dissolution performance in both SD and PM complexes. These promising results support the use of specific co-solvents with CD as a new raw material for insoluble compounds. The studied solid dispersions showed promising features to improve BNZ efficacy against Chagas disease.




Similar content being viewed by others
References
Sharmin N, Rudd CD (2017) Structure, thermal properties, dissolution behaviour and biomedical applications of phosphate glasses and fibres: a review. J Mater Sci 52:8733–8760. doi:10.1007/s10853-017-0784-4
Saini S, Quinot D, Lavoine N, Belgacem MN, Bras J (2017) β-Cyclodextrin-grafted TEMPO-oxidized cellulose nanofibers for sustained release of essential oil. J Mater Sci 52:3849–3861. doi:10.1007/s10853-016-0644-7
Vartiainen V, Bimbo LM, Hirvonen J, Kauppinen EI, Raula J (2017) Aerosolization, drug permeation and cellular interaction of dry powder pulmonary formulations of corticosteroids with hydroxypropyl-β-cyclodextrin as a solubilizer. Pharm Res 34:25–35
Anandam S, Selvamuthukumar S (2014) Fabrication of cyclodextrin nanosponges for quercetin delivery: physicochemical characterization, photostability, and antioxidant effects. J Mater Sci 49:8140–8153. doi:10.1007/s10853-014-8523-6
Lopedota A, Cutrignelli A, Laquintana V, Denora N, Iacobazzi RM, Perrone M, Fanizza E, Mastrodonato M, Mentino D, Lopalco A, Depalo N, Franco M (2016) Spray dried chitosan microparticles for intravesical delivery of celecoxib: preparation and characterization. Pharm Res 33:2195–2208
Loftsson T, Brewster ME (2012) Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J Pharm Sci 101:3019–3032
Medarevíc D, Kachrimanis K, Djuríc Z, Ibríc S (2015) Influence of hydrophilic polymers on the complexation of carbamazepine with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci 78:273–285
Vieira ACC, Fontes DAF, Chaves LL, Alves LDS, Freitas Neto JL, Soares JL, Soares-Sobrinho MFLR, Rolim LA, Rolim-Neto PJ (2015) Multicomponent systems with cyclodextrins and hydrophilic polymers for the delivery of Efavirenz. Carbohyd Polym 130:133–140
Chibunova ES, Kumeev RS, Terekhova IV (2015) Thermodynamic study on salt effects on complex formation of α-, β- and γ-cyclodextrins with p-aminobenzoic acid. J Chem Thermodyn 91:30–34
Terekhova I, Chibunova E, Kumeev R, Kruchinin S, Fedotova M, Kozbiał M, Wszelaka-Rylik M, Gierycz P (2015) Specific and nonspecific effects of biologically active inorganic salts on inclusion complex formation of cyclodextrins with aromatic carboxylic acids. Chem Eng Sci 122:97–103
Aiassa V, Zoppi A, Albesa I, Longhi MR (2015) Inclusion complexes of chloramphenicol with β-cyclodextrin and aminoacids as a way to increase drug solubility and modulate ROS production. Carbohyd Polym 121:320–327
Banchero M, Manna L (2012) The use of lysine to enhance the supercritical complexation of ketoprofen and cyclodextrins. J Supercrit Fluid 67:76–83
Higashi K, Ideura S, Limwikrant W, Moribe K, Yamamoto K (2010) Simultaneous dissolution of naproxen and flurbiprofen from a novel ternary γ-cyclodextrin complex. Chem Pharm Bull 58:769–772
Gamer AO, Rossbacher R, Kaufmann W, Ravenzwaay B (2008) The inhalation toxicity of di- and triethanolamine upon repeated exposure. Food Chem Toxicol 46:2173–2183
Rowe RC, Sheskey PJ, Quinn ME (2009) Handbook of pharmaceutical excipients, 6th edn. Pharmaceutical Press, London, pp 601–755
Granero G, Garnero C, Longhi M (2003) The effect of pH and triethanolamine on sulfisoxazole complexation with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci 20:285–293
Mora MJ, Tártara LI, Onnainty R, Palma SD, Longhi MR, Granero GE (2013) Characterization, dissolution and in vivo evaluation of solid acetazolamide complexes. Carbohyd Polym 98:380–390
Garnero C, Longhi M (2007) Study of ascorbic acid interaction with hydroxypropyl-β-cyclodextrin and triethanolamine, separately and in combination. J Pharmaceut Biomed 45:536–545
Miyako Y, Khalef N, Matsuzaki K, Pinal R (2010) Solubility enhancement of hydrophobic compounds by cosolvents: role of solute hydrophobicity on the solubilization effect. Int J Pharm 393:48–54
Jain P, Yalkowsky SH (2007) Solubilization of poorly soluble compounds using 2-pyrrolidone. Int J Pharm 342:1–5
Medeiros ASA, Zoppi A, Barbosa EG, Oliveira JIN, Fernandes-Pedrosa MF, Longhi MR, Silva-Júnior AA (2016) Supramolecular aggregates of oligosaccharides with co-solvents in ternary systems for the solubilizing approach of triamcinolone. Carbohyd Polym 20:1040–1051
Dias LC, Dessoy MA (2009) Quimioterapia da doença de chagas: estado da arte e perspectivas no desenvolvimento de novos fármacos (Chemotherapy of Chagas’ disease: state of the art and perspectives for the development of new drugs), Quim. Nova 32:2444–2457 (in Portuguese)
Hall BS, Wilkinson SR (2012) Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob Agents Chemother 56:115–123
Maximiano FP, Costa GHY, Souza J, Cunha-Filho MSS (2010) Physicochemical characterization of antichagasic benznidazole, Quim. Nova 33:1714–1719 (in Portuguese)
DNDi—Drugs for Neglected Disease initiative (2017) http://www.dndial.org/pt/tratamentos/benznidazol-pediatrico.html. Accessed 13 Mar 2017
Salomon CJ (2012) First century of Chagas’ disease: an overview on novel approaches to nifurtimox and benznidazole delivery systems. J Pharm Sci 101:888–894
Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Arment IA (2009) Side effects of benznidazole as treatment in chronic Chagas’ disease: fears and realities. Expert Rev Anti Infect Ther 7:157–163
Paula FR, Jorge SD, Almeida LV, Pasqualoto KFM, Tavares LC (2009) Molecular modeling studies and in vitro bioactivity evaluation of a set of novel 5-nitro-heterocyclic derivatives as anti-T. cruzi agents. Bioorgan Med Chem 17:2673–2679
Bernardes LSC, Zani CL, Carvalho I (2013) Trypanosomatidae diseases: from the current therapy to the efficacious role of trypanothione reductase in drug discovery. Curr Med Chem 20:2673–2696
Fonseca-Berzal C, Palmeiro-Roldán R, Escario JA, Torrado S, Arán VJ, Torrado-Santiago S, Gómez-Barrio A (2015) Novel solid dispersions of benznidazole: preparation, dissolution profile and biological evaluation as alternative antichagasic drug delivery system. Exp Parasitol 149:84–91
Streck L, Sarmento VHV, Machado PRL, Farias KJS, Fernandes-Pedrosa MF, Silva-Júnior AA (2016) Phase transitions of isotropic to anisotropic biocompatible lipid-based drug delivery systems overcoming insoluble benznidazole loading. Int J Mol Sci 17:981–998
Leonardi D, Bombardiere ME, Salomon CJ (2013) Effects of benznidazole:cyclodextrin complexes on the drug bioavailability upon oral administration to rats. Int J Biol Macromol 62:543–548
Soares-Sobrinho JL, Santos FLA, Lyra MAM, Alves LDS, Rolim LA, Lima AAN, Nunes LCC, Soares MFR, Rolim-Neto PJ, Torres-Labandeira JJ (2012) Benznidazole drug delivery by binary and multicomponent inclusion complexes using cyclodextrins and polymers. Carbohyd Polym 89:323–330
Melo PN, Barbosa EG, Caland LB, Carpegianni H, Garnero C, Longhi M, Fernandes-Pedrosa MF, Silva-Júnior AA (2013) Host-guest interactions between benznidazole and beta-cyclodextrin in multicomponent complex systems involving hydrophilic polymers and triethanolamine in aqueous solution. J Mol Liq 186:147–156
Melo PN, Barbosa EG, Garnero C, Caland LB, Fernandes-Pedrosa MF, Longhi MR, Silva-Júnior AA (2016) Interaction pathways of specific co-solvents with hydroxypropyl-β-cyclodextrin inclusion complexes with benznidazole in liquid and solid phase. J Mol Liq 223:350–359
U.S. FDA. Dissolution testing of immediate release solid oral dosage forms, guidance for industry, Food and Drug Administration. Center Drug Evaluation and Research. (CDER) (1997) Rockville, USA, pp 1–10
USP 30-NF 25. 30th revision of the United States Pharmacopeia and 25th edition of National Formulary (2007) United States Pharmacopeial Convention. Rockville, USA
Saini GSS, Kaur S, Tripathi SK, Dogra SD, Abbas JM, Mahajan CG (2011) Vibrational spectroscopic and density functional theory studies of chloranil–imidazole interaction. Vib Spectrosc 56:66–73
Mohan PRK, Sreelakshmi G, Muraleedharan CV, Joseph R (2012) Water soluble complexes of curcumin with cyclodextrins: characterization by FT-Raman spectroscopy. Vib Spectrosc 62:77–84
D’Sa DJ, Lechuga-Ballesteros D, Chan HK (2015) Isothermal microcalorimetry of pressurized systems II: effect of excipient and water ingress on formulation stability of amorphous glycopyrrolate. Pharm Res 32:714–722
Zhang D, Zhang J, Jiang K, Li K, Cong Y, Pu S, Jin Y, Lin J (2016) Preparation, characterisation and antitumour activity of β-, γ- and HP-β-cyclodextrin inclusion complexes of oxaliplatin. Spectrochim Acta A 152:501–508
Oliveira AR, Molina EF, Mesquita PC, Fonseca JLC, Rossanezi G, Fernandes-Pedrosa MF, Oliveira AG, Silva-Júnior AA (2013) Structural and thermal properties of spray-dried methotrexate-loaded biodegradable microparticles. J Therm Anal Calorim 112:555–565
Aigner Z, Berkesi O, Farkas G, Szabó-Révész P (2012) Dsc, X-ray and FTIR studies of a gemfibrozil/dimethyl-β-cyclodextrin inclusion complex produced by co-grinding. J Pharmaceut Biomed 57:62–67
Dandawate PR, Vyas A, Ahmad A, Banerjee S, Deshpande J, Swamy KV, Jamadar A, Dumhe-Klaire AC, Padhye S, Sarkar FH (2012) Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic. Cancer Pharm 29:1775–1786
Figueiras A, Carvalho RA, Ribeiro L, Torres-Labandeira JJ, Veiga FJB (2007) Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified β-cyclodextrin. Eur J Pharm Biopharm 67:531–539
Naidu NB, Chowdary KPR, Murthy KVR, Satyanarayana KVR, Hayman AR, Becket G (2004) Physicochemical characterization and dissolution properties of meloxicam–cyclodextrin binary systems. J Pharmaceut Biomed 35:75–86
Maria DN, Abd-Elgawad AEH, Soliman OAE, El-dahan MS, Jablonski MM (2017) Nimodipine ophthalmic formulations for management of glaucoma. Pharm Res 34:809–824
Wang L, Yan J, Li Y, Xu K, Li S, Tang P, Li H (2016) The influence of hydroxypropyl-β-cyclodextrin on the solubility, dissolution, cytotoxicity, and binding of riluzole with human serum albumin. J Pharmaceut Biomed 117:453–463
Cho AR, Chun YG, Kim BK, Park DJ (2014) Preparation of alginate–CaCl2 microspheres as resveratrol carriers. J Mater Sci 49:4612–4619
Cho W, Kim MS, Jung MS, Park J, Cha KH, Kim JS, Park HJ, Alhalaweh A, Velaga SP, Hwang SJ (2015) Design of salmon calcitonin particles for nasal delivery using spray-drying and novel supercritical fluid-assisted spray-drying processes. Int J Pharm 478:288–296
Mesquita PC, Oliveira AR, Pedrosa MFF, Oliveira AG, Silva-Júnior AA (2015) Physicochemical aspects involved in methotrexate release kinetics from biodegradable spray-dried chitosan microparticles. J Phys Chem Solids 81:27–33
Kadota K, Nishimura T, Hotta D, Tozuka Y (2015) Preparation of composite particles of hydrophilic or hydrophobic drugs with highly branched cyclic dextrin via spray drying for dry powder inhalers. Powder Technol 283:16–23
Loftsson T, Duchêne D (2007) Cyclodextrins and their pharmaceutical applications. Int J Pharm 329:1–11
Naito Y, Matsuda H, Shimomura K, Kurihara K, Tochigi K, Tomono K (2013) Measurement and correlation of solubilities of the poorly water-soluble pharmaceutical compound etodolac by addition of co-solvents. Fluid Phase Equilib 357:43–49
Brewster ME, Loftsson T (2007) Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliver Rev 59:645–666
Mura P, Bettinetti GP, Manderioli A, Faucci MT, Bramanti G, Sorrenti M (1998) Interactions of ketoprofen and ibuprofen with b-cyclodextrins in solution and in the solid state. Int J Pharm 166:189–203
Miletic T, Kyriakos K, Graovac A, Ibric S (2013) Spray-dried voriconazole–cyclodextrin complexes: solubility, dissolution rate and chemical stability. Carbohyd Polym 98:122–130
Acknowledgements
The authors wish to thank the Brazilian National Council for Scientific and Technological Development (CNPq) for the financial support (Grant Nos. 483073/2010-5; 481767/2012-6) and the Coordination for the Improvement of Graduation Students (CAPES) (Grant No. AUXPE no. PNPD 23038.007487/2011-91 and scholarship of de Melo, P.N. and de Caland, LB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Melo, P.N., de Caland, L.B., Fernandes-Pedrosa, M.F. et al. Designing and monitoring microstructural properties of oligosaccharide/co-solvent ternary complex particles to improve benznidazole dissolution. J Mater Sci 53, 2472–2483 (2018). https://doi.org/10.1007/s10853-017-1720-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1720-3