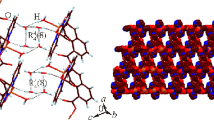
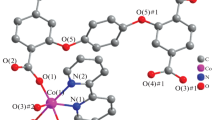
Abstract
The zwitterionic dicarboxylate 1,1′-[(2,3,5,6-tetramethylbenzene-1,4-diyl)bis(methylene)]bis(pyridin-1-ium-4-carboxylate) (L) has been reacted with uranyl nitrate under solvo-hydrothermal conditions and in the presence of KReO4 to give the complex [UO2(L)(OH)(H2O)](ReO4) (1). This compound crystallizes as a cationic, monoperiodic coordination polymer with ReO4– as a simple counterion. The daisy-chain polymer is based on dinuclear rings built by the convergent zwitterionic ligands, these rings being linked to one another by double hydroxide bridges. In addition to a Coulombic interaction with a pyridinium ring, ReO4– is involved in one OH(water)⋅⋅⋅O and four CH⋅⋅⋅O interactions, and it is thus nestled in a cavity formed by three chains, seemingly with some selectivity over nitrate and chloride anions also present in the reaction mixture. This result illustrates the interest of zwitterionic dicarboxylates in building cationic assemblies able to trap ReO4–, a surrogate for the radioactive TcO4–, an anion of environmental relevance.
Graphical abstract
A zwitterionic dicarboxylate ligand was reacted with the uranyl ion to generate a cationic, daisychain coordination polymer including perrhenate as counterion. Examination of the Hirshfeld surface of the anion allows for an analysis of the weak interactions involved.




Similar content being viewed by others
Data availability
Crystallographic data have been deposited with the CCDC under number 2311986.
References
Andrews, M.B., Cahill, C.L.: Uranyl bearing hybrid materials: synthesis, speciation, and solid-state structures. Chem. Rev. 113, 1121–1136 (2013). https://doi.org/10.1021/cr300202a
Loiseau, T., Mihalcea, I., Henry, N., Volkringer, C.: The crystal chemistry of uranium carboxylates. Coord. Chem. Rev. 266–267, 69–109 (2014). https://doi.org/10.1016/j.ccr.2013.08.038
Su, J., Chen, J.S.: MOFs of uranium and the actinides. Struct. Bonding 163, 265–296 (2015)
Thuéry, P., Harrowfield, J.: Recent advances in structural studies of heterometallic uranyl-containing coordination polymers and polynuclear closed species. Dalton Trans. 46, 13660–13667 (2017). https://doi.org/10.1039/C7DT03105J
Lv, K., Fichter, S., Gu, M., März, J., Schmidt, M.: An updated status and trends in actinide metal-organic frameworks (An-MOFs): from synthesis to application. Coord. Chem. Rev. 446, 214011 (2021). https://doi.org/10.1016/j.ccr.2021.214011
Wang, Y., Liu, Z., Li, Y., Bai, Z., Liu, W., Wang, Y., Xu, X., Xiao, C., Sheng, D., Diwu, J., Su, J., Chai, Z., Albrecht-Schmitt, T.E., Wang, S.: Umbellate distortions of the uranyl coordination environment result in a stable and porous polycatenated framework that can effectively remove cesium from aqueous solutions. J. Am. Chem. Soc. 137, 6144–6147 (2015). https://doi.org/10.1021/jacs.5b02480
Hu, K.Q., Jiang, X., Wang, C.Z., Mei, L., Xie, Z.N., Tao, W.Q., Zhang, X.L., Chai, Z.F., Shi, W.Q.: Solvent-dependent synthesis of porous anionic uranyl–organic frameworks featuring a highly symmetrical (3,4)-connected ctn or bor topology for selective dye adsorption. Chem. Eur. J. 23, 529–532 (2017). https://doi.org/10.1002/chem.201604225
Ai, J., Chen, F.Y., Gao, C.Y., Tian, H.R., Pan, O.J., Sun, Z.M.: Porous anionic uranyl–organic networks for highly efficient Cs+ adsorption and investigation of the mechanism. Inorg. Chem. 57, 4419–4426 (2018). https://doi.org/10.1021/acs.inorgchem.8b00099
Liu, W., Xie, J., Zhang, L., Silver, M.A., Wang, S.: A hydrolytically stable uranyl organic framework for highly sensitive and selective detection of Fe3+ in aqueous media. Dalton Trans. 47, 649–653 (2018). https://doi.org/10.1039/C7DT04365A
Hu, F., Di, Z., Lin, P., Huang, P., Wu, M., Jiang, F., Hong, M.: An anionic uranium-based metal–organic framework with ultralarge nanocages for selective dye adsorption. Cryst. Growth Des. 18, 576–580 (2018). https://doi.org/10.1021/acs.cgd.7b01525
Zeng, L.W., Hu, K.Q., Mei, L., Li, F.Z., Huang, Z.W., An, S.W., Chai, Z.F., Shi, W.Q.: Structural diversity of bipyridinium-based uranyl coordination polymers: synthesis, characterization, and ion-exchange application. Inorg. Chem. 58, 14075–14084 (2019). https://doi.org/10.1021/acs.inorgchem.9b02106
Kong, X.H., Hu, K.Q., Mei, L., Li, A., Liu, K., Zeng, L.W., Wu, Q.Y., Chai, Z.F., Nie, C.M., Shi, W.Q.: Double-Layer Nitrogen-Rich Two-Dimensional Anionic Uranyl–Organic Framework forCation Dye Capture and Catalytic Fixation of Carbon Dioxide. Inorg. Chem. 60, 11485–11495 (2021). https://doi.org/10.1021/acs.inorgchem.1c01492
Baruah, J.B.: Coordination polymers in adsorptive remediation of environmental contaminants. Coord. Chem. Rev. 470, 214694 (2022). https://doi.org/10.1016/j.ccr.2022.214694
Banerjee, D., Kim, D., Schweiger, M.J., Kruger, A.A., Thallapally, P.K.: Removal of TcO4– ions from solution: materials and future outlook. Chem. Soc. Rev. 45, 2724–2739 (2016). https://doi.org/10.1039/C5CS00330J
Bai, Z., Wang, Y., Li, Y., Liu, W., Chen, L., Sheng, D., Diwu, J., Chai, Z., Albrecht- Schmitt, T.E., Wang, S.: First cationic uranyl–organic framework with anion-exchange capabilities. Inorg. Chem. 55, 6358–6360 (2016). https://doi.org/10.1021/acs.inorgchem.6b00930
Banerjee, D., Xu, W., Nie, Z., Johnson, L.E.V., Coghlan, C., Sushko, M.L., Kim, D., Schweiger, M.J., Kruger, A.A., Doonan, C.J., Thallapally, P.K.: Zirconium-based metal–organic framework for removal of perrhenate from water. Inorg. Chem. 55, 8241–8243 (2016). https://doi.org/10.1021/acs.inorgchem.6b01004
Zhu, L., Xiao, C., Dai, X., Li, J., Gui, D., Sheng, D., Chen, L., Zhou, R., Chai, Z., Albrecht-Schmitt, T.E., Wang, S.: Exceptional perrhenate/pertechnetate uptake and subsequent immobilization by a low-dimensional cationic coordination polymer: overcoming the Hofmeister bias selectivity. Environ. Sci. Technol. Lett. 4, 316–322 (2017). https://doi.org/10.1021/acs.estlett.7b00165
Rapti, S., Diamantis, S.A., Dafnomili, A., Pournara, A., Skliri, E., Armatas, G.S., Tsipis, A.C., Spanopoulos, I., Malliakas, C.D., Kanatzidis, M.G., Plakatouras, J.C., Noli, F., Lazarides, T., Manos, M.J.: Exceptional TcO4– sorption capacity and highly efficient ReO4– luminescence sensing by Zr4+ MOFs. J. Mater. Chem. A. 6, 20813–20821 (2018). https://doi.org/10.1039/C8TA07901C
Li, C.P., Ai, J.Y., Zhou, H., Chen, Q., Yang, Y., He, H., Du, M.: Ultra-highly selective trapping of perrhenate/pertechnetate by a flexible cationic coordination framework. Chem. Commun. 55, 1841–1844 (2019). https://doi.org/10.1039/C8CC09364D
Brunet, G., Robeyns, K., Huynh, R.P.S., Lin, J.B., Collins, S.P., Facey, G.A., Shimuzu, G.K.H., Woo, T.K., Murugesu, M.: Design strategy for the controlled generation of cationic frameworks and ensuing anion-exchange capabilities. ACS Appl. Mater. Interfaces 11, 3181–3188 (2019). https://doi.org/10.1021/acsami.8b15946
Li, C.P., Zhou, H., Chen, J., Wang, J.J., Du, M., Zhou, W.: A highly efficient coordination polymer for selective trapping and sensing of perrhenate/pertechnetate. ACS Appl. Mater. Interfaces 12, 15246–15254 (2020). https://doi.org/10.1021/acsami.0c00775
John, G.H., May, I., Sarsfield, M.J., Steele, H.M., Collison, D., Helliwell, M., McKinney, J.D.: The structural and spectroscopic characterisation of three actinyl complexes with coordinated and uncoordinated perrhenate: [UO2(ReO4)2(TPPO)3], [{(UO2)(TPPO)3}2(µ2-O2)][ReO4]2 and [NpO2(TPPO)4][ReO4]. Dalton Trans. (2004). https://doi.org/10.1039/B313045B
Bühl, M., Golubnychiy, V.: Binding of pertechnetate to uranyl(VI) in aqueous solution. A density functional theory molecular dynamics study. Inorg. Chem. 46, 8129–8131 (2007). https://doi.org/10.1021/ic701431u
Chaumont, A., Klimchuk, O., Gaillard, C., Billard, I., Ouadi, A., Hennig, C., Wipff, G.: Perrhenate Complexation by Uranyl in Traditional solvents and in ionic liquids: A joint Molecular Dynamics/Spectroscopic Study. J. Phys. Chem. B. 116, 3205–3219 (2012). https://doi.org/10.1021/jp209476h
Sutton, A.D., John, G.H., Sarsfield, M.J., Renshaw, J.C., May, I., Martin, L.R., Selvage, A.J., Collison, D., Helliwell, M.: Spectroscopic evidence for the direct coordination of the Pertechnetate Anion to the Uranyl Cation in [UO2(TcO4)(DPPMO2)2]+. Inorg. Chem. 43, 5480–5482 (2004). https://doi.org/10.1021/ic049588r
Sarsfield, M.J., Sutton, A.D., May, I., John, G.H., Sharrad, C., Helliwell, M.: Coordination of pertechnetate [TcO4]– to actinides. Chem. Commun. (2004). https://doi.org/10.1039/B404424J
Budantseva, N., Andreev, G., Fedoseev, A.: Structural role of isonicotinic acid in U(VI), Np(VI), and Pu(VI) complexes with TcO4–, ReO4–, and ClO4– ions. Inorg. Chem. 56, 12199–12205 (2017). https://doi.org/10.1021/acs.inorgchem.7b01611
John, G.H., May, I., Sarsfield, M.J., Collison, D., Helliwell, M.: Dimeric uranyl complexes with bridging perrhenates. Dalton Trans. (2007). https://doi.org/10.1039/B614481K
Karimova, O.V., Burns, P.C.: Structural units in three uranyl perrhenates. Inorg. Chem. 46, 10108–10113 (2007). https://doi.org/10.1021/ic701128b
Thuéry, P.: Uranyl-lanthanide heterometallic complexes with cucurbit[6]uril and perrhenate ligands. Inorg. Chem. 48, 825–827 (2009). https://doi.org/10.1021/ic802270n
Thuéry, P., Masci, B.: Uranyl ion complexation by cucurbiturils in the presence of perrhenic, phosphoric, or polycarboxylic acids. novel mixed-ligand uranyl–organic frameworks. Cryst. Growth Des. 10, 716–725 (2010). https://doi.org/10.1021/cg901121c
Andreev, G., Budantseva, N., Fedoseev, A.: The novel U(VI) and Np(VI) mixed-ligand complexes based on quinaldic acid zwitterion. Polyhedron. 224, 116003 (2022). https://doi.org/10.1016/j.poly.2022.116003
Thuéry, P.: Uranyl ion complexes of cucurbit[7]uril with zero-, one- and two-dimensionality. CrystEngComm. 11, 1150–1156 (2009). https://doi.org/10.1039/B822872H
Thuéry, P.: Lanthanide complexes with cucurbit[n]urils (n = 5, 6, 7) and perrhenate ligands: new examples of encapsulation of perrhenate anions. Inorg. Chem. 48, 4497–4513 (2009). https://doi.org/10.1021/ic900328z
Thuéry, P.: Second-sphere complexation of thorium(IV) by cucurbit[6]uril with included perrhenate counterions–crystal structure and Hirshfeld surface analysis. Eur. J. Inorg. Chem. (2015). https://doi.org/10.1002/ejic.201500171
Farrell, D., Gloe, K., Gloe, K., Goretzki, G., McKee, V., Nelson, J., Nieuwenhuyzen, M., Pál, I., Stephan, H., Town, R.M., Wichmann, K.: Towards promising oxoanion extractants: azacages and open-chain counterparts. Dalton Trans. (2003). https://doi.org/10.1039/B210289G
Stephan, H., Gloe, K., Kraus, W., Spies, H., Johannsen, B., Wichmann, K., Reck, G., Chand, D.K., Bharadwaj, P.K., Müller, U., Müller, W.M., Vögtle, F.: In: Moyer, B.A., Singh, R.P. (eds.) Fundamentals and applications of anion separation, pp. 151–168. Kluwer Academic/Plenum, New York (2004)
Katayev, E.A., Kolesnikov, G.V., Sessler, J.L.: Molecular recognition of pertechnetate and perrhenate. Chem. Soc. Rev. 38, 1572–1586 (2009). https://doi.org/10.1039/B806468G
Amendola, V., Alberti, G., Bergamaschi, G., Biesuz, R., Boiocchi, M., Ferrito, S., Schmidtchen, F.P.: Cavity effect on perrhenate recognition by polyammonium cages. Eur. J. Inorg. Chem. (2012). https://doi.org/10.1002/ejic.201200334
Alberto, R., Bergamaschi, G., Braband, H., Fox, T., Amendola, V.: 99TcO4–: selective recognition and trapping in aqueous solution. Angew Chem. Int. Ed. 51, 9772–9776 (2012). https://doi.org/10.1002/anie.201205313
Amendola, V., Bergamaschi, G., Boiocchi, M., Alberto, R., Braband, H.: Fluorescent sensing of 99Tc pertechnetate in water. Chem. Sci. 5, 1820–1826 (2014). https://doi.org/10.1039/C3SC53504E
Burke, B.P., Grantham, W., Burke, M.J., Nichol, G.S., Roberts, D., Renard, I., Hargreaves, R., Cawthorne, C., Archibald, S.J., Lusby, P.J.: Visualizing kinetically robust CoIII4L6 assemblies in vivo: SPECT Imaging of the encapsulated [99mTc]TcO4– anion. J. Am. Chem. Soc. 140, 16877–16881 (2018). https://doi.org/10.1021/jacs.8b09582
Thevenet, A., Marie, C., Tamain, C., Amendola, V., Miljkovic, A., Guillaumont, D., Boubals, N., Guilbaud, P.: Perrhenate and pertechnetate complexation by an azacryptand in nitric acid medium. Dalton Trans. 49, 1446–1455 (2020). https://doi.org/10.1039/C9DT04314D
Xu, L., Zhang, D., Ronson, T.K., Nitschke, J.R.: Improved acid resistance of a metal–organic cage enables cargo release and exchange between hosts. Angew Chem. Int. Ed. 59, 7435–7438 (2020). https://doi.org/10.1002/ange.202001059
Thevenet, A., Miljkovic, A., La Cognata, S., Marie, C., Tamain, C., Boubals, N., Mangano, C., Amendola, V., Guilbaud, P.: Syntheses and evaluation of new hydrophilic azacryptands used as masking agents of technetium in solvent extraction processes. Dalton Trans. 50, 1620–1630 (2021). https://doi.org/10.1039/D0DT04210B
Zhang, D., Gan, Q., Plajer, A.J., Lavendomme, R., Ronson, T.K., Lu, Z., Jensen, J.D., Laursen, B.W., Nitschke, J.R.: Templation and concentration drive conversion between a FeII12L12 pseudoicosahedron, a FeII4L4 tetrahedron, and a FeII2L3 helicate. J. Am. Chem. Soc. 144, 1106–1112 (2022). https://doi.org/10.1021/jacs.1c11536
Baklouti, L., Harrowfield, J.: Oligozwitterions in coordination polymers and frameworks – a structural view. Dalton Trans. 52, 7772–7786 (2023). https://doi.org/10.1039/D3DT00849E
Li, F.Z., Geng, J.S., Hu, K.Q., Yu, J.P., Liu, N., Chai, Z.F., Mei, L., Shi, W.Q.: Proximity effect in uranyl coordination of the cucurbit[6]uril-bipyridinium pseudorotaxane ligand for promoting host–guest synergistic chelating. Inorg. Chem. 60, 10522–10534 (2021). https://doi.org/10.1021/acs.inorgchem.1c01177
Berger, M., Schmidtchen, F.P.: Zwitterionic guanidinium compounds serve as electroneutral anion hosts. J. Am. Chem. Soc. 121, 9986–9993 (1999). https://doi.org/10.1021/ja992028k
Cresswell, A.L., Piepenbrock, M.O.M., Steed, J.W.: A water soluble, anion-binding zwitterionic capsule based on electrostatic interactions between self-complementary hemispheres. Chem. Commun. 46, 2787–2789 (2010). https://doi.org/10.1039/B926149D
Wenzel, M., Bruere, S.R., Knapp, Q.W., Tasker, P.A., Plieger, P.G.: Zwitterionic dicopper helicates: anion encapsulation and binding studies. Dalton Trans. 39, 2936–2941 (2010). https://doi.org/10.1039/B922998A
Kusumoto, S., Atoini, Y., Masuda, S., Kim, J.Y., Hayami, S., Kim, Y., Harrowfield, J., Thuéry, P.: Zwitterionic and anionic polycarboxylates as coligands in uranyl ion complexes, and their influence on periodicity and topology. Inorg. Chem. 61, 15182–15203 (2022). https://doi.org/10.1021/acs.inorgchem.2c02426
Kusumoto, S., Atoini, Y., Masuda, S., Koide, Y., Kim, J.Y., Hayami, S., Kim, Y., Harrowfield, J., Thuéry, P.: Varied role of organic carboxylate dizwitterions and anionic donors in mixed-ligand uranyl ion coordination polymers. CrystEngComm. 24, 7833–7844 (2022). https://doi.org/10.1039/D2CE01187E
APEX3, ver: 2019.1-0, Bruker AXS, Madison, WI, (2019)
SAINT: ver. 8.40A, Bruker Nano, Madison, WI, (2019)
SADABS: 2016/2, Bruker AXS, Madison, WI, (2016)
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., Stalke, D.: Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 48, 3–10 (2015). https://doi.org/10.1107/S1600576714022985
Sheldrick, G.M.: SHELXT - integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A. 71, 3–8 (2015). https://doi.org/10.1107/S2053273314026370
Sheldrick, G.M.: Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C. 71, 3–8 (2015). https://doi.org/10.1107/S2053229614024218
Hübschle, C.B., Sheldrick, G.M., Dittrich, B.: ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44, 1281–1284 (2011). https://doi.org/10.1107/S0021889811043202
Burnett, M.N., Johnson, C.K.: ORTEPIII, Report ORNL-6895; Oak Ridge National Laboratory. TN (1996)
Farrugia, L.J.: WinGX and ORTEP for windows: an update. J. Appl. Crystallogr. 45, 849–854 (2012). https://doi.org/10.1107/S0021889812029111
Momma, K., Izumi, F.: VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011). https://doi.org/10.1107/S0021889811038970
Thuéry, P., Harrowfield, J.: Uranyl ion complexes with 2,2′:6′,2′′-terpyridine-4′-carboxylate. Interpenetration of networks involving expanded ligands. CrystEngComm. 23, 7305–7313 (2021). https://doi.org/10.1039/D1CE01215K
Thuéry, P., Harrowfield, J.: Varying structure-directing anions in uranyl ion complexes with Ni(2,2′: 6′,2′′-terpyridine-4′-carboxylate)2. Eur. J. Inorg. Chem. (2022). https://doi.org/10.1002/ejic.202200011
Kusumoto, S., Atoini, Y., Masuda, S., Koide, Y., Kim, J.Y., Hayami, S., Kim, Y., Harrowfield, J., Thuéry, P.: Flexible aliphatic diammonioacetates as Zwitterionic ligands in UO22+ complexes: diverse topologies and interpenetrated structures. Inorg. Chem. 62, 3929–3946 (2023). https://doi.org/10.1021/acs.inorgchem.2c04321
Kusumoto, S., Atoini, Y., Koide, Y., Chainok, K., Hayami, S., Kim, Y., Harrowfield, J., Thuéry, P.: Nanotubule inclusion in the channels formed by a six-fold interpenetrated, triperiodic framework. Chem. Commun. 59, 10004–10007 (2023). https://doi.org/10.1039/D3CC02636A
Kavallieratos, K., Moyer, B.A.: Attenuation of Hofmeister bias in ion-pair extraction by a disulfonamide anion host used in strikingly effective synergistic combination with a calix-crown Cs+ host. Chem. Commun. (2001). https://doi.org/10.1039/B102152B
Moyer, B.A., Bonnesen, P.V., Custelcean, R., Delmau, L.H., Hay, B.P.: Strategies for using host-guest chemistry in the extractive separations of ionic guests. Kem Ind. 54, 65–87 (2005). https://doi.org/10.1002/chin.200531276
Custelcean, R., Moyer, B.A.: Anion separation with metal–organic frameworks. Eur. J. Inorg. Chem. (2007). https://doi.org/10.1002/ejic.200700018
Etter, M.C., MacDonald, J.C., Bernstein, J.: Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B. 46, 256–262 (1990). https://doi.org/10.1107/S0108768189012929
Bernstein, J., Davis, R.E., Shimoni, L., Chang, N.L.: Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew Chem. Int. Ed. 34, 1555–1573 (1995). https://doi.org/10.1002/anie.199515551
Spek, A.L.: Structure validation in chemical crystallography. Acta Crystallogr. Sect. D. 65, 148–155 (2009). https://doi.org/10.1107/S090744490804362X
Wolff, S.K., Grimwood, D.J., McKinnon, J.J., Turner, M.J., Jayatilaka, D., Spackman, M.A. CrystalExplorer, University of Western Australia (2012)
Spackman, P.R., Turner, M.J., McKinnon, J.J., Wolff, S.K., Grimwood, D.J., Jayatilaka, D., Spackman, M.A.: CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 54, 1006–1011 (2021). https://doi.org/10.1107/S1600576721002910
Ahuja, R., Samuelson, A.G.: Non-bonding interactions of anions with nitrogen heterocycles and phenyl rings: a critical Cambridge Structural database analysis. CrystEngComm 5, 395–399 (2003). https://doi.org/10.1039/B311000A
Schottel, B.L., Chifotides, H.T., Dunbar, K.R.: Anion-π interactions. Chem. Soc. Rev. 37, 68–83 (2008). https://doi.org/10.1039/B614208G
Hay, B.P., Custelcean, R.: Anion – π interactions in crystal structures: commonplace or extraordinary? Cryst. Growth Des. 9, 2539–2545 (2009). https://doi.org/10.1021/cg900308b
Berryman, O.B., Johnson, D.W.: Experimental evidence for interactions between anions and electron-deficient aromatic rings. Chem. Commun. (2009). https://doi.org/10.1039/B823236A
Gamez, P.: The anion–π interaction: naissance and establishment of a peculiar supramolecular bond. Inorg. Chem. Front. 1, 35–43 (2014). https://doi.org/10.1039/C3QI00055A
Frontera, A.: Encapsulation of anions: macrocyclic receptors based on metal coordination and anion–π interactions. Coord. Chem. Rev. 257, 1716–1727 (2013). https://doi.org/10.1016/j.ccr.2013.01.032
Bauzá, A., Mooibroek, T.J., Frontera, A.: Towards design strategies for anion–π interactions in crystal engineering. CrystEngComm. 18, 10–23 (2016). https://doi.org/10.1039/C5CE01813G
Giese, M., Albrecht, M., Rissanen, K.: Experimental investigation of anion–π interactions – applications and biochemical relevance. Chem. Commun. 52, 1778–1795 (2016). https://doi.org/10.1039/C5CC09072E
Das, A., Choudhury, S.R., Estarellas, C., Dey, B., Frontera, A., Hemming, J., Helliwell, M., Gamez, P., Mukhopadhyay, S.: Supramolecular assemblies involving anion–π and lone pair–π interactions: experimental observation and theoretical analysis. CrystEngComm 13, 4519–4527 (2011). https://doi.org/10.1039/C0CE00593B
Fiol, J.J., Barceló-Oliver, M., Tasada, A., Frontera, A., Terrón, A., García-Raso, A.: Structural characterization, recognition patterns and theoretical calculations of long-chain N-alkyl substituted purine and pyrimidine bases as ligands: on the importance of anion–π interactions. Coord. Chem. Rev. 257, 2705–2715 (2013). https://doi.org/10.1016/j.ccr.2012.12.013
Thuéry, P., Atoini, Y., Harrowfield, J.: Tubelike uranyl–phenylenediacetate assemblies from screening of ligand isomers and structure-directing counterions. Inorg. Chem. 58, 6550–6564 (2019). https://doi.org/10.1021/acs.inorgchem.9b00804
Thuéry, P., Atoini, Y., Harrowfield, J.: 1,2-, 1,3-, and 1,4-Phenylenediacetate complexes of the uranyl ion with additional metal cations and/or ancillary N-donor ligands: confronting ligand geometrical proclivities. Cryst. Growth Des. 19, 6611–6626 (2019). https://doi.org/10.1021/acs.cgd.9b01032
Thuéry, P., Atoini, Y., Harrowfield, J.: Structure-directing effects of coordinating solvents, ammonium and phosphonium counterions in uranyl ion complexes with 1,2-, 1,3-, and 1,4-phenylenediacetates. Inorg. Chem. 59, 2503–2518 (2020). https://doi.org/10.1021/acs.inorgchem.9b03404
Thuéry, P., Harrowfield, J.: Stepwise introduction of flexibility into aromatic dicarboxylates forming uranyl ion coordination polymers: a comparison of 2-carboxyphenylacetate and 1,2-phenylenediacetate. Eur. J. Inorg. Chem. (2021). https://doi.org/10.1002/ejic.202100203
Acknowledgements
This work was supported by KAKENHI Grant-in-Aid for Early-Career Scientists JP22K14698 for S. Kusumoto, and JSPS KAKENHI Grant Number 20K21213 for S. Hayami.
Author information
Authors and Affiliations
Contributions
SK, YA, YK, SH, YK and PT contributed to the investigation. JH and PT wrote the main manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kusumoto, S., Atoini, Y., Koide, Y. et al. Perrhenate anion encapsulation in a uranyl ion–zwitterionic dicarboxylate coordination polymer. J Incl Phenom Macrocycl Chem (2024). https://doi.org/10.1007/s10847-024-01238-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10847-024-01238-0