Abstract
Lipoic acid derivative of cyclodextrin, βCDLip, was used as the drug carrier for doxorubicin (DOX) and the stability constants of the DOX–βCDLip were determined in the environment of the cell medium. The experiments were performed in neutral (pH 7.6) and acidified (pH 6.3) cell media containing more than forty interferences including: amino acids, vitamins, lipids and proteins. We proved that the pH of the medium has a noticeable impact on the affinity of the drug towards the carrier. At neutral pH, the formation constants of the complex are higher than at pH 6.3, what is characteristic for the cancer cells microenvironment. Furthermore, the values obtained in both cell media are twice smaller than the values obtained previously for the same complex but in the absence of common cell media components indicating that there is a competition between DOX and some hydrophobic medium components for the complex formation with βCDLip. On the other hand at pH 7.6, the amount of free DOX is highly limited due to the fact that most of DOX is still in the complexed form, while at pH 6.3 the cell media ingredients become strong interferences in the formation of the complex between DOX and the drug carrier. The observed behaviour is due to partial protonation of DOX and to competition between the drug and the lipoic side arm of cyclodextrin for the cyclodextrin cavity. The stability constants of the DOX–βCDLip complex in acidic pH are similar to the values for DOX with native β-cyclodextrin, demonstrating that the strengthening effect of DOX–CD complex resulting from the presence of cyclodextrin’s aromatic substituent (Lip) occurs only in the case of neutral pH. The high value of the stability constant of the DOX–βCDLip complex in cell medium at pH 7.6 indicates high selectivity of βCDLip ligand which would be of importance both for the effective drug delivery and for its application in DOX sensing devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs) are cyclic organic compounds obtained by the enzymatic transformation of starch. They possess a hydrophilic exterior and a hydrophobic cavity able to host various guest compounds bound via noncovalent bonds to form inclusion complexes [1,2,3]. The inclusion ability of CDs has attracted considerable attention due to its multiple applications in sensing devices, molecular machines and drug delivery of aromatic drugs such as anthracyclines [4]. Doxorubicin (DOX, Adriamycin®), antibiotic isolated from Streptomyces peucetius var. caesius [5], is one of the most effective drugs in this group. Thanks to the wide spectrum of action in both experimental cancer models and human malignancies, this drug has been used clinically for over 50 years. DOX molecule contains a weakly basic amino sugar, daunosamine, linked via a glycosidic bond to the red-pigmented tetracyclic moiety, adriamycinone. It is used for the treatment of several types of cancers, such as lung [6], breast [7], cervix [8], ovarian [9], prostate [10] and brain cancers [11]. The drug effect primarily consists in the modification of the DNA structure as a result of intercalation complex formation assisted with covalent binding. In addition, DOX inhibits topoisomerase II, increasing the stability of the drug-enzyme-DNA complex, and prevents DNA repair [12,13,14]. The clinical application of DOX has been limited by serious adverse effects [15, 16]. The specific toxicity of the drug is due to reactive oxygen species (ROS) produced by redox reactions of anthracyclines creating radical oxygen species. ROS can interact with cellular components such as proteins, lipids, carbohydrates or nucleic acids [17, 18]. All of these reactions can cause damage and, in case of DNA, mutations. Cardiotoxicity is the most disturbing and dangerous effect of anthracyclines [19]. It is believed that the anthracyclines induce cardiotoxicity through different mechanisms from those responsible for their anti-cancer activity, and research has shown that a decreased amount of reactive oxygen species leads to the reduction of the cardiotoxic effects of anthracyclines [20]. Due to the fact, that the quinone group of anthracycline is responsible for ROS production, the protection of this group until the drug is delivered to the cancer cell gives the opportunity to minimize the cardiotoxic effect of DOX. This protective effect can be achieved by placing the anthracycline molecule in the hydrophobic cavity of cyclodextrin (CD).
Previous studies have shown that DOX molecule fits into the cavity of CD from the quinone side, however the stability constant of the anthracycline-CD complex is rather low [21]. We have shown in our previous reports that this difficulty can be eliminated by the modification of cyclodextrin with an appropriate functional group, such as aromatic substituent or triazole moiety and lipoic acid [22,23,24,25]. Aromatic triazole linker can significantly increase the stability of the DOX–CD complex through π–π interactions occurring between triazole linker of CD and the aglicone group of the drug. Additionally, cyclodextrin containing a triazole linker is sensitive towards pH and supports binding of the drug to DNA [22, 26]. In a recent study, we described the synthesis and complexing abilities of a new derivative of cyclodextrin possessing lipoic acid connected via triazole linker (βCDLip). The lipoic acid is a naturally occurring antioxidant compound, whose protective properties are ascribed to the ability of sulphur atoms to scavenge reactive oxygen species. It was found to break the chain reaction of the biomolecule oxidation [27]. The derivative of cyclodextrin described here could be employed for pH-controlled binding of anthracycline drugs. While at pH 7.4 the new derivative formed strong complex with DOX, at pH 5.5 the stability constant was orders of magnitude smaller. Molecular modelling of the lipoic acid-cyclodextrin conjugate (βCDLip) alone and in the presence of DOX allowed to prove that at pH 5.5, a self-inclusion complex is formed with the lipoic acid substituent located inside the cavity of cyclodextrin [22]. The competition between the formation of the DOX–βCDLip inclusion complex and the βCDLip self-inclusion complex is the reason why at pH 5.5, the drug is easily released from cyclodextrin cavity, whereas at pH 7.4, it remains inside the cavity - self-inclusion of the lipoic acid substituent is not favored at this pH. In addition, the protonation of the anthracycline molecule facilitates the drug release at lower pH. The influence of pH is confirmed by a more negative value of the interaction energy of the βCDLip self-inclusion complex at pH 5.5. Therefore, this ligand can be useful as potential drug carrier in cancer treatment since it allows for the release of the drug in more acidic environment, corresponding to that of the cancer cell while holding it firmly at neutral pH corresponding to healthy cells. Unfortunately, the physicochemical parameters of this and similar drug-cyclodextrin complexes were usually evaluated in simple electrolyte solutions. The data obtained in this manner cannot be, however, a reliable basis for any predictions concerning the utility of respective cyclodextrins as drug carriers. The composition of the biological matrix, which can obviously influence the physicochemical data, has to be taken into account. The components of the biological media employed for the cytotoxicity assays will affect the composition and stability of the drug-CD complexes. Amino acids especially those with aromatic side chains [28, 29], lipids [30] or cholesterol [31] and vitamins [32, 33], have also affinity to the cyclodextrin cavity and can displace the drug from the inclusion complex, changing the ratio of the free drug to its complexed form. Reaction with cholesterol can e.g. affect the integrity of membranes and introduce additional negative effects when using cyclodextrins as the complexing agents [34]. It is, therefore, important to evaluate the stability of the drug-CD complex in the multicomponent environment of the biological media.
In the present study, our aim is to describe the influence of the cell medium on the stability of DOX complex with lipoic acid derivative of cyclodextrin and to determine the formation constants of these complexes at the pH values of normal and cancer affected cells.
Experimental
Chemicals and reagents
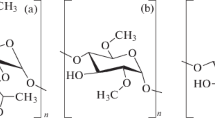
Doxorubicin hydrochloride salt was purchased from LC Laboratories (Woburn, USA). Other compounds used in this work were purchased from Aldrich, Fluka and Thermo Fisher Scientific. The pH was measured using a pH-Meter E2 (Mettler Toledo). Lipoic acid derivatives of cyclodextrin (βCDLip), Scheme 1, were synthesized as described in our recent report, [22].
All measurements were performed in a mixture of RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), penicillin (100 u/ml; Thermo Fisher Scientific), streptomycin (100 µg/ml; Thermo Fisher Scientific) and 25 mM HEPES (Thermo Fisher Scientific), pH 7.6 or 25 mM HEPES and 25 mM HCl (PPH Standard), pH 6.3.
Methods
Electrochemical measurements
Linear sweep voltammetry (LSV) experiments were performed using an OGF500 potentiostat (OrigaFlex). All electrochemical experiments were performed in a three-electrode arrangement with a silver/silver chloride (Ag/AgCl/saturated KCl) reference electrode (BASi), platinum foil as the counter electrode, and a glassy carbon electrode (GCE, BASi, 3 mm diameter) as the working electrode. The working electrode was polished mechanically with 1.0-, 0.3-, and 0.05-µm alumina powder on a Buehler polishing cloth, washed with 0.5M sulphuric acid (H2SO4) several times and polished again with 0.05 µm alumina powder. Next, the working electrode was rinsed with water and used in LSV experiments. The cell medium components strongly adsorb on GC electrode, therefore, the cleaning procedure was repeated before each LSV scan. The concentration of DOX was 5 × 10− 5 M, whereas the concentration of βCDLip was varied from 2.50 × 10− 4 to 1.75 × 10− 3 M.
Due to the presence of hydroquinone and quinone groups in DOX molecule, the drug can be either electrooxidized or reduced, as shown in Scheme 2.
The reduction process takes place at negative potentials and is affected by the presence of oxygen, hence the measurements have to be carried out under anaerobic conditions. The electrooxidation process takes place at positive potentials, thus does not require prior deoxygenation of the solution, what significantly simplifies the measurements, especially in media of such complex composition and high viscosity as those reported here.
To calculate the formation constants of the DOX–CDLip complexes, the modification of Osa Eq. (1) for diffusion-controlled processes were used: [35]
where Iobs is the oxidation peak current and IDOX and IDOX−CD are the oxidation peak currents for the free DOX and the inclusion complex, respectively. Ks is the complex formation constant, and CCD is the concentration of cyclodextrin.
The value of Ks was calculated from the slope of the linear plot of Iobs2 vs. (IDOX2 − Iobs2)/ CCD.
Results and discussion
Electrochemical measurements
Reproducible quantitative electrochemical analysis of the drug/drug-carrier complex in a natural multi-component matrix, such as cell medium, is complicated because of adsorption of the components and drug itself on the electrode surface, causing partial blocking of the electrode and decrease of the analytical peak. Therefore, a cleaning procedure of the working electrode before each measurement with 0.5 M sulphuric acid (as described above) has to be used to obtain reproducible results with a low standard deviation and linearity of the calibration plot (Fig. 1b).
a Voltammetric oxidation curves for 5.0 × 10− 5 M DOX in cell medium, at pH 7.6 (1) and 6.3 (2). b Calibration curve for DOX in cell medium at pH 7.6. All potentials reported vs. silver/silver chloride (Ag/AgCl) electrode. Scan rate 100 mV/s. c Dependence of the peak current on the square root of scan rate
Linear sweep voltammograms showed well-developed DOX oxidation peaks recorded in solutions at pH 7.6 and 6.3 (Fig. 1a). Linear dependence of the oxidation peak current on the square root of scan rate confirmed diffusion-control of the electrode process (Fig. 1c).
As shown in our previous study on DOX–CDLip complex in pure electrolyte solutions, there is a clear competition between the formation of the DOX–βCDLip inclusion complex and the βCDLip self-inclusion complex. At acidic pH, self-inclusion of the lipoic acid side arm in the cyclodextrin cavity dominates. The complex is stronger than that with protonated, hence charged DOX. However, at neutral pH, self-inclusion of the lipoic acid substituent is not favoured, and the complexation of DOX by the cyclodextrin takes place (Scheme 3). This behaviour is observed also in the cell medium.
Addition of βCDLip to DOX in the cell medium solution resulted in the decrease of the voltammetric peak currents due to the decrease of diffusion coefficient of the DOX–βCDLip complex compared with that of the free guest (Fig. 2).
The dependencies of DOX oxidation peak current on βCDLip concentration recorded in cell medium at pH 7.6 and 6.3 are shown in Fig. 3a, b). The formation constants of the DOX–βCDLip complex were calculated using Eq. 1. The dependencies of I2obs on (I2DOX - I2obs)/CCD for βCDLip at pH 7.6 and pH 6.3 obtained using LSV method are shown in Fig. 3c, d.
The dependencies of DOX oxidation peak current on βCDLip concentration (a, b) and Osa plots (Eq. 1) for DOX in the presence of βCDLip (c, d), recorded in cell media at pH 7.6 (a, c) and 6.3 b, d. Scan rate 100 mV/s. All potentials reported vs. (Ag/AgCl) reference electrode
Formation of the inclusion complex between DOX and βCDLip in the cell medium is also confirmed by the shift of peak potentials towards more positive values as a result of the change in the drug environment to a more hydrophobic one (cyclodextrin cavity), Fig. 4.
The values of formation constants determined for the DOX–βCDLip complex are 4.20 ± 0.45 × 103 M− 1 and 0.70 ± 0.31 × 103 M− 1 in cell media of pH 7.6 and 6.3, respectively. At pH 7.6, corresponding to the pH of body fluids or neutral medium, the formation constants of the complex are higher than those at pH 6.3, which is characteristic for cancer cells microenvironment. The values obtained in both cell media are ca. twice smaller at pH 7.6, and four times smaller at pH 6.3 than the values obtained previously for the same complex [22] in pure electrolyte solutions (7.80 ± 0.63 × 103 M− 1 at neutral pH, and 2.7 ± 0.4 × 103 M− 1 at acidic pH). This is expected, since the medium contains several compounds able to form inclusion complexes with cyclodextrins e.g. aromatic amino acids (such as l-phenylalanine, l-tryptophan, l-tyrosine), vitamins (biotin, choline, folic acid, niacinamide, B12, riboflavin, thiamine, para-aminobenzoic acid, pyridoxine hydrochloride) and other minor components. The lower values of formation constants determined for the DOX–βCDLip complex indicate that there is a competition between DOX and hydrophobic medium components for the βCDLip cavity. At pH 7.6, the amount of free DOX is lower than in pure electrolyte solution due to the fact that most of DOX is in the complexed form. At pH 6.3 the stability constants of the DOX–βCDLip complex are similar to the value obtained for DOX with non-modified (native) β-cyclodextrin. This result confirms that the effect of cyclodextrin aromatic substituent which strengthens the DOX–βCDLip complex is important in cell medium conditions only at neutral pH. Previous studies conducted in pure electrolyte solutions have shown that the lipoic acid substituent present in DOX–βCDLip strengthens the complexes with DOX both at lower and neutral pH as compared to those of pristine βCD. The high value of the stability constant of the DOX–βCDLip complex at pH 7.6 observed in the cell medium indicates that the βCDLip ligand shows high affinity to the drug molecules even in the multicomponent biological environment. This is very important for the application of this ligand as the drug carrier and in addition it opens the possibilities of using it in cyclodextrin modified electrodes for monitoring drug levels in biological samples, environmental waste solutions containing the antibiotic and, particularly, in biological fluids. The 1,2-dithiolane ring (-S-S-) group would allow binding of the ligand to the surface of both the metallic electrodes and nanoparticles. Research in this direction is carried out in our laboratory.
Conclusions
In this study we examined the influence of cell medium on the stability constants of DOX complex with a potential drug carrier-the lipoic acid derivative of βCD. The lipoic acid derivative of βCD was chosen since it was shown to increase the stability of the complex compared with that of pristine CD in synthetic solutions. This behaviour is due to capping effect of the substituent as shown in Scheme 3. The experiments in this work were carried out in the cell media at neutral (pH 7.6) and acidified (pH 6.3). The cell media contained more than forty compounds including: amino acids, vitamins, lipids and proteins which could interfere in the DOX–βCDLip complex formation. We showed that the pH of the medium has a noticeable impact on the binding strength of the drug by the hosting ligand leading to decreased values of conditional stability constants of the complexes. At pH 7.6, corresponding to the pH of body fluids as well as in pure neutral supporting electrolyte solutions, the formation constants of the complex are higher than those at pH 6.3, which is characteristic for cancer cells microenvironment. At pH 7.6, the amount of free DOX exposed to the medium environment is limited since most of DOX is in the complexed form, while at pH 6.3 the stability constants of the DOX–βCDLip complex are lower and similar to those obtained for DOX with non-modified (native) β-cyclodextrin. This shows that in acidified solutions the drug is more exposed to the influence of other components of the biological medium and the protecting role of βCDLip ligand used in the present paper is weaker than in pure electrolyte solutions.
The high value of the stability constant of the DOX–βCDLip complex at pH 7.6 retained in the cell medium indicates high selectivity of βCDLip for DOX pointing to its potential utility not only as the drug delivery system but also as the receptor molecule in new electroanalytical devices monitoring levels of DOX in biological fluids and waste solutions of this toxic chemotherapeutic. The presence of the 1,2-dithiolane ring (-S-S-) in the βCDLip derivative will facilitate stable immobilization of the ligand on metallic e.g. gold electrodes and gold nanoparticles.
References
Szejtli, J., Atwood, J.L., Lehn, J.M.: Comprehensive supramolecular chemistry. Elsevier, New York (1996)
Dodziuk, H.: Rigidity versus flexibility. A review of experimental and theoretical studies pertaining to the cyclodextrin nonrigidity. J. Mol. Struct. 614, 33–45 (2002). https://doi.org/10.1016/S0022-2860(02)00236-3
Kaifer, A., Gmez-Kaifer, M.: Supramolecular Electrochemistry, pp. i–xiv. Wiley, Weinheim (1999)
Bilewicz, R., Chmurski, K.: Chap. 10: other physicochemical methods, Chap. 16: applications other than in the pharmaceutical industry. In: Cyclodextrins and Their Complexes, Chemistry, Analytical Methods, Applications, pp. 255–332. Wiley, Weinheim (2006)
Arcamone, F., Franceschi, G., Penco, S., Selva, A.: Adriamycin (14-hydroxydaunomycin), a novel antitumor antibiotic. Tetrahedron Lett. 10, 1007–1010 (1969). https://doi.org/10.1016/S0040-4039(01)97723-8
Hong, Y., Che, S., Hui, B., Yang, Y., Wang, X., Zhang, X., Qiang, Y., Ma, H.: Lung cancer therapy using doxorubicin and curcumin combination: targeted prodrug based, pH sensitive nanomedicine. Biomed. Pharmacother. 112, 108614 (2019). https://doi.org/10.1016/j.biopha.2019.108614
Christowitz, C., Davis, T., Isaacs, A., van Niekerk, G., Hattingh, S., Engelbrecht, A.M.: Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer. 19, 757 (2019). https://doi.org/10.1186/s12885-019-5939-z
Omura, G.A., Hubbard, J., Hatch, K.: Chemotherapy of cervix cancer with doxorubicin and cisplatin. A phase I pilot study of the gynecologic oncology group. Am. J. Clin. Oncol. 8, 347–349 (1985). https://doi.org/10.1097/00000421-198510000-00001
Pisano, C., Cecere, S.C., Di Napoli, M., Cavaliere, C., Tambaro, R., Facchini, G., Scaffa, C., Losito, S., Pizzolorusso, A., Pignata, S.: Clinical trials with pegylated liposomal doxorubicin in the treatment of ovarian cancer. J. Drug. Deliv. 2013, 898146 (2013)
Fan, X., Wang, L., Guo, Y., Xiong, X., Zhu, L., Fang, K.: Inhibition of prostate cancer growth using doxorubicin assisted by ultrasound-targeted nanobubble destruction. Int. J. Nanomed. 11, 3585–3596 (2016). https://doi.org/10.2147/IJN.S111808
MacDiarmid, J.A., Langova, V., Bailey, D., Pattison, S.T., Pattison, S.L., Christensen, N., Armstrong, L.R., Brahmbhatt, V.N., Smolarczyk, K., Harrison, M.T., Costa, M., Mugridge, N.B., Sedliarou, I., Grimes, N.A., Kiss, D.L., Stillman, B., Hann, C.L., Gallia, G.L., Graham, R.M., Brahmbhatt, H.: Targeted doxorubicin delivery to brain tumors via minicells: proof of principle using dogs with spontaneously occurring tumors as a model. PLoS ONE 11, e0151832 (2016). https://doi.org/10.1371/journal.pone.0151832
Beretta, G.L., Zunino, F.: Molecular Mechanisms of Anthracycline Activity. In: Krohn, K. (ed.) Anthracycline Chemistry and Biology II: Mode of Action, Clinical Aspects and New Drugs, pp. 1–19. Springer-Verlag GmbH, Heidelberg (2008)
Bains, O.S., Szeitz, A., Lubieniecka, J.M., Cragg, G.E., Grigliatti, T.A., Riggs, K.W., Reid, R.E.: A correlation between cytotoxicity and reductase-mediated metabolism in cell lines treated with doxorubicin and daunorubicin. J. Pharmacol. Exp. Ther. 347, 375–387 (2013). https://doi.org/10.1124/jpet.113.206805
Quigley, G.J., Wang, A.H., Ughetto, G., van der Marel, G., van Boom, J.H., Rich, A.: Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). PNAS 77, 7204–7208 (1980). https://doi.org/10.1073/pnas.77.12.7204
Takemura, G., Fujiwara, H.: Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 49, 330–352 (2007). https://doi.org/10.1016/j.pcad.2006.10.002
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., Gianni, L.: Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 56, 185–229 (2004). https://doi.org/10.1124/pr.56.2.6
Raghunand, N., Gillies, R.J.: pH and drug resistance in tumors. Drug Resist. Updat. 3, 39–47 (2000). https://doi.org/10.1054/drup.2000.0119
Rahbari, R., Sheahan, T., Modes, V., Collier, P., Macfarlane, C., Badge, R.M.: A novel L1 retrotransposon marker for HeLa cell line identification. Biotechniques 46, 277–284 (2009). https://doi.org/10.2144/000113089
Zucchi, R., Danesi, R.: Cardiac toxicity of antineoplastic anthracyclines. Anti-Cancer Agents Med. Chem. 3, 151–171 (2003). https://doi.org/10.2174/1568011033353434
Myers, C.E., McGuire, W., Young, R.: Adriamycin: amelioration of toxicity by alpha-tocopherol. Cancer Treat Rep. 60, 961–962 (1976)
Husain, N., Ndou, T.T., De La Peña, A.M., Warner, I.M.: Complexation of doxorubicin with β and γ-cyclodextrins. Appl Spectrosc. 46, 652–658 (1992). https://doi.org/10.1366/0003702924124943
Swiech, O., Majdecki, M., Debinski, A., Krzak, A., Stępkowski, T.M., Wójciuk, G., Kruszewski, M., Bilewicz, R.: Competition between self-inclusion and drug binding explains the pH dependence of the cyclodextrin drug carrier—molecular modelling and electrochemistry studies. Nanoscale 8, 16733–16742 (2016). https://doi.org/10.1039/C6NR05833G
Majdecki, M., Krzak, A., Zelechowska, K., Swiech, O.: Monosubstituted hydrazone β-cyclodextrin derivatives for pH-sensitive complex formation with aromatic drugs. J. Incl. Phenom. Macrocycl. Chem. 93, 77–83 (2019). https://doi.org/10.1007/s10847-018-0841-x
Swiech, O., Dutkiewicz, P., Wójciuk, K., Chmurski, K., Kruszewski, M., Bilewicz, R.: Cyclodextrin derivatives conjugated with aromatic moieties as pH-responsive drug carriers for anthracycline. J. Phys. Chem. B 117, 13444–13450 (2013). https://doi.org/10.1021/jp4060632
Swiech, O., Mieczkowska, A., Chmurski, K., Bilewicz, R.: Intermolecular interactions between doxorubicin and β-cyclodextrin 4-methoxyphenol conjugates. J. Phys. Chem. B 116, 1765–1771 (2012). https://doi.org/10.1021/jp2091363
Krzak, A., Swiech, O., Majdecki, M., Bilewicz, R.: Complexing daunorubicin with b-cyclodextrin derivative increases drug intercalation into DNA. Electrochim. Acta 247, 139–148 (2017). https://doi.org/10.1016/j.electacta.2017.06.140
Fuchs, J. (ed.): Lipoic Acid in Health and Disease. CRC Press, New York (1997)
Mn, R.: Probing inclusion complexes of cyclodextrins with amino acids by physicochemical approach. Carbohydr. Polym. 151, 458–466 (2016). https://doi.org/10.1016/j.carbpol.2016.05.100
Roy, M.N., Ekka, D., Saha, S., Roy, M.C.: Host–guest inclusion complexes of α and β-cyclodextrins with α-amino acids. RSC Adv. 4, 42383–42390 (2014). https://doi.org/10.1039/C4RA07877B
Szente, L., Fenyvesi, É.: Cyclodextrin-lipid complexes: cavity size matters. Struct. Chem. 28, 479–492 (2017). https://doi.org/10.1007/s11224-016-0884-9
Ravichandran, R., Divakar, S.: Inclusion of ring A of cholesterol inside the β-cyclodextrin cavity: evidence from oxidation reactions and structural studies. J. Incl. Phenom. 30, 253–270 (1998). https://doi.org/10.1023/A:1007912809965
Comini, S., Olivier, P., Riottot, M., Duhamel, D.: Interaction of β-cyclodextrin with bile acids and their competition with vitamins A and D3 as determined by 1H-NMR spectrometry. Clin. Chim. Acta. 228, 181–194 (1994). https://doi.org/10.1016/0009-8981(94)90288-7
Palmieri, G.F., Wehrlé, P., Duportail, G., Stamm, A.: Inclusion complexation of vitamin a palmitate with β-cyclodextrin in aqueous solution. Drug. Dev. Ind. Pharm. 18, 2117–2121 (1992). https://doi.org/10.3109/03639049209040925
Vecsernyés, M., Fenyvesi, F., Bácskay, I., Deli, M.A., Szente, L., Fenyvesi, É.: Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch. Med. Res. 45, 711–729 (2014). https://doi.org/10.1016/j.arcmed.2014.11.020
Osa, T., Matsue, T., Fujihira, M.: Cyclodextrin—nitrophenol systems studied by polarography. Heterocycles 6, 1833–1839 (1977)
Acknowledgements
The project was financed by the Polish National Science Centre No. 2016/23/B/ST4/03295. O.S. thanks also the National Science Centre for the support through Project No. 2011/01/N/ST5/05550.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Swiech, O., Majdecki, M., Opuchlik, L.J. et al. Impact of pH and cell medium on the interaction of doxorubicin with lipoic acid cyclodextrin conjugate as the drug carrier. J Incl Phenom Macrocycl Chem 97, 129–136 (2020). https://doi.org/10.1007/s10847-020-00994-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-00994-z