Abstract
A new and convenient synthetic pathway was developed to produce monosubstituted cyclodextrins with high yields. Each of the β-cyclodextrin derivatives described in this work has an aromatic substituent connected with cyclodextrin core by a pH-sensitive hydrazone linker and a carbon chain. Carbon chains differ in lengths having one or three carbon atoms. The correlation between water solubility and linker length was determined using UV–Vis spectroscopy, while the dependence of hydrazone bond hydrolysis on the electrolyte pH was confirmed by cyclic voltammetry. The pH-dependent complex-formation ability between the hydrazone derivative of cyclodextrin and anthracycline drug was examined by square wave voltammetry. The significantly big solubility and the appropriate pH, at which the hydrolysis of the hydrazone bond occurs, make the newly synthesized derivatives attractive for pharmaceutical and medical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The selective synthesis of the monosubstituted β-cyclodextrins (βCDs) is a challenge for chemists. Native β-cyclodextrin consists of seven glucopyranose units, so the total number of hydroxyl groups in this molecule is equal to 21. The presence of three types of hydroxyl groups at two edges of cyclodextrin molecule requires appropriate methods to obtain the substitution of only one hydroxyl group in the chosen position.
Of the three types of hydroxyl groups present in the βCD, the hydroxyl groups at 6th position (primary hydroxyls) are the most basic and generally most nucleophilic. The hydroxyl groups at the 2nd position are the most acidic, while groups at the 3rd position are the most inaccessible. Such diversity makes it possible to perform selective functionalization, however in most cases, it is extremely difficult. As the primary hydroxyl groups (those at 6th positions) are the most nucleophilic, they are also most sensitive to the attack of electrophiles, and the modification takes place preferentially at these positions. However, not only monosubstitution, but also higher order substitution, up to persubstitution at 6th position takes place. The most popular method for the monosubstitution at 6th position of CD consists of two steps, involving tosylation of CD and nucleophilic displacement of tosyl moiety by other group. These mono-tosyl derivatives (6-O-Monotosyl-β-cyclodextrins) are obtained in the reaction of stoichiometric amounts of p-toluenesulfonic chloride with CD dissolved in pyridine or DMF containing base. However, this procedure suffers from difficulty of the post-synthetic purification, as several by-products, including complexes, are formed. The overall yields are relatively low and lie between 15 and 30%. Replacement of harmful pyridine with aqueous alkaline medium results in obtaining 6-O-Monotosyl-β-cyclodextrins of acceptable purity and in fairly good yields [1, 2].
Despite the drawbacks of 6-O-Monotosyl-β-cyclodextrins preparation procedures, they are still under interest and their improvements appear continuously in the literature. Tosylated CD can be converted into other derivatives e.g. monoamino CD, monoalkyl or aryl CD and others. Tosyl CD was also oxidized using DMSO to produce monoaldehydic cyclodextrin [3, 4].
A more facile procedure of obtaining CD derivatives, in which CD monoaldehyde was synthesized using iodoxybenzoic acid (Dess-Martin periodinane), giving c.a. 90% yield, was proposed by Cornwell et al. [5,6,7].
Monoaldehydic CD provides a route for further modification, for example hydroxylamine or hydrazine can react with its carbonyl group to produce monooxime or monohydrazone derivatives. It is known, that hydrazone bond is stable at neutral pH, but falls apart under acidic conditions. We present here the synthesis of monofunctionalized CD with a pH-sensitive bond connected to aromatic group by linkers of two different lengths. The ending moiety is the methoxyphenol derivative and it was chosen as an example of the aromatic ring with increased electron density. Thus, it may stabilize CD complexes with compounds possessing aromatic residues with decreased electron density, such as anthracycline antibiotic—doxorubicin. In such case π–π interactions between aromatic rings of host and guest are enhanced, leading to the formation of more stable complexes, as we have shown in our previous studies [8, 9].
Doxorubicin (DOX), an anthracycline antibiotic, is one of the most commonly used anticancer agents for the treatment of several types of cancers, such as lung, breast, ovarian, prostate, and brain cancers. Clinical effects of the drug are ascribed to modification of the DNA structure primarily through formation of intercalating complexes and covalent bonds [10,11,12]. However, the clinical application of DOX has been limited by serious adverse effects [13, 14]. The specific toxicity of the drug results from the presence of reactive oxygen species (ROS), produced by redox reactions of anthracyclines, creating radical oxygen species that interact with cellular components such as proteins, lipids, carbohydrates or nucleic acids. The most disturbing and dangerous effect of anthracyclines is their cardiotoxicity. It is believed that the anthracyclines induce cardiotoxicity through different mechanisms than those responsible for its anti-cancer activity and research has shown that a decreased amount of reactive oxygen species leads to the reduction of the cardiotoxic effects of anthracyclines [15].
To prevent the adverse effects of reactive oxygen species, quinone groups of anthracycline, responsible for their production, can be blocked until the drug molecule is delivered to the pathologically changed cells. This blocking effect can be achieved by formation of a complex between the anthracycline molecule and a cyclodextrin. Previous studies of the anthracycline–CD complexes have shown that doxorubicin molecule fits into the cavity of the CD from the quinone side [16]. However, this approach is limited by low stability constant of the CD–drug complex [17]. This can be solved by the modification of cyclodextrin with an appropriate functional aromatic group, such as p-methoxy-phenyl derivative, which can significantly increase the stability of the CD–DOX complex [8].
The most common pharmaceutical application of cyclodextrins is using them to increase the solubility of the drug in aqueous solutions [18,19,20,21]. However, pristine βCD exhibits low water solubility, thus chemical modification is required. The effect of cyclodextrins presence on the chemical stability of drugs is another useful property of these excipients and it has been extensively described in the literature [22,23,24]. Controlled drug release from a CD cavity is the key factor to their usage in medicine, including anti-cancer therapy.
In this work we synthesized new, well water-soluble derivatives of cyclodextrin, possessing aromatic substituent containing a pH-sensitive hydrazone linker. The dependence of the hydrazone bond hydrolisis on pH changes was examined by cyclic voltammetry, while the ability of the cyclodextrin to formation of inclusion complex with doxorubicin, in neutral and acidic pH, was determined using square wave voltammetry technique.
Experimental section
Reagents and apparatus
All reagents and solvents were purchased from Sigma-Aldrich and used without further purification. Buffers were prepared using water from a Milli-Q ultrapure water system.
Britton–Robinson (BR) buffer (pH 3–12) was prepared by the addition of appropriate amounts of 0.2 M sodium hydroxide to orthophosphoric acid, acetic acid, and boric acid (0.04 M solutions). The pH was measured using an E2 pH meter (Mettler Toledo). NMR spectra were conducted using INOVA spectrophotometer with 500 MHz frequency. UV–Visible spectroscopic measurements were performed using an EVOLUTION60 spectrophotometer with a 1-cm acrylic cell. Time of flight Mass Spectrometer LCT (TOF) was used to identify synthesized compounds. Electrochemical measurements were performed using an EC Epsilon potentiostat (BASI).
Synthesis of hydrazone derivatives of cyclodextrins: CD_1 and CD_2—general procedure
Syntheses of 4-methoxyphenoxy acetic hydrazide and 4-methoxyphenoxy butyric acid hydrazide were performed according to the previously reported literature procedure [25]. The monoaldehyde of CD was obtained using procedure described by Cornwell et al. [5].
Cyclodextrin monoaldehyde and respective hydrazides (4-methoxyphenoxy acetic hydrazide or 4-methoxyphenoxy butyric hydrazide) were used to produce respective hydrazone derivatives of cyclodextrins, Scheme 1. 1 g (0.88 mmol) of cyclodextrin monoaldehyde was dissolved in 10 ml of DMSO and 4-methoxyphenoxy acetic or butyric acid hydrazide (1.25 mmol) was added. The mixture was stirred overnight at room temperature and then 50 ml of acetone was added. After cooling to 0 °C, flurry precipitate was formed. It was filtered on the Büchner funnel and dried under reduced pressure. CD-hydrazones were obtained as golden-yellow solids.
βCD_1: Yield 89%. 1H NMR (500 MHz; d-DMSO) δ: 11.40 (s, ~ 1H); 6.99–6.80 (m, 4H); 5.85–5.65 (m, 14H); 4.86–4.80 (m, 7H); 4.48–4.40 (m, 8H); 3.78–3.48 (m, 29H); 3.42–3.25 (m, 7H).
βCD_2. Yield 91%. 1H NMR (500 MHz, DMSO) δ 6.83 (s, 4H), 5.65 (bs, 7H), 5.04–4.73 (m, 6H), 3.99–3.05 (m, 61H), 1.91 (bs, 2H), Fig. S1.
13C NMR (126 MHz, d-DMSO) δ 153.4 (C=O), 115.58, 115.37, 114.58, 114.50 (Ph), 101.91 (C1), 81.52 (C4), 73.02–72.01 (C2, C3, C5), 67.40 (O–CH2), 59.89 (C-6), 55.29 (O–CH3), 30.63 (CH2CH2–CH2), 21.37 (CH2–CH2–CH2), Fig. S2.
HRMS (ES): Calc. for 1361.4494 C53H82N2O37Na ([M + Na]), found 1361.4479, Fig. S3.
Electrochemical Measurements were performed using an EC Epsilon potentiostat (BASI). All electrochemical experiments were performed in a three-electrode arrangement with a silver/silver chloride (Ag/AgCl) electrode (BASI) in a saturated solution of KCl as the reference electrode, platinum foil as the counter electrode, and glassy carbon electrode (BASI, 3-mm diameter) as the working electrode. The working electrode was polished mechanically with 1.0-, 0.3-, and 0.05-µm alumina powder on a Buehler polishing cloth. For performing electrochemical measurements of hydrolysis process, the solutions of CD_2 in water were prepared as follows: βCD solution (0.05 M) was initially prepared in DMSO. Then 6 µl of as-prepared solution was added to 3 ml of the appropriate buffer. The mixture was left for 24 h at 4 °C. Prior to measurements, the buffer solutions were purged with purified argon for 10 min, and all experiments were performed at room temperature. Experiments in DMSO were performed with addition of a 0.1 M solution of tetrabutylammonium hexafluorophosphate. For the stability constants determination the square wave voltammetry (SWV) technique was used. Before each SWV run, the electrode was electrochemically cleaned by applying a negative potential of − 1.0 V for 40 s, as described by Mora et al. [26]. The experiment was carried out by varying the potential between − 0.2 and − 0.9 V, at a frequency of 25 Hz, with an amplitude of 25 mV and a step equal to 2 mV. The concentration of DOX was 1 × 10−6 M, whereas the concentration of CD_2 varied from 1.0 × 10−5 to 1.5 × 10−4 M.
DOX is electroactive (Scheme 2), and therefore square wave voltammetry results could be employed to calculate the formation constant of the CD_2–DOX complex based on the Osa equation [27]:
where Iobs is the observed reduction peak current, and IDOX and IβCD−DOX are the reduction peak currents for free DOX and the inclusion complex, respectively. Ks is the complex formation constant, and CβCD is the concentration of β-cyclodextrin. The value of Ks was calculated from the slope of the linear plot of Iobs2 versus (IDOX2 − Iobs2)/CβCD.
Solubility measurements
Saturated solutions of cyclodextrin derivatives were prepared by dissolving 60 mg of CD_1 or CD_2 in 0.5 ml of ultra-pure water. The solutions were mixed in ultrasound bath for 15 min and left to decant for 24 h. After this time the supernatant was diluted: 10, 25, 50, 75 and 100 times and UV–Vis spectra were collected. The values of molar absorptivity, ε, were determined from a series of standard solutions and equaled to 1213 ± 32 dm3 mol−1 cm−1 and 1609 ± 13 dm3 mol−1 cm−1 for CD_1 and CD_2, respectively.
Results and discussion
Synthesis and characterization of CD derivatives
βCD monoaldehyde was obtained with yield higher than 90% and was characterized by 1H NMR and FTIR spectroscopies. The NMR spectrum revealed presence of a signal at δ 9.7 referring to a single hydrogen in the aliphatic aldehyde group. The formation of aldehyde was also confirmed by FTIR spectroscopy, as a characteristic band for carbonyl group appeared at 1710 cm−1, Fig. S4. Monoaldehyde derivative of βCD was converted into βCD-hydrazones in the reaction with respective hydrazides, as it is shown in Scheme 1. Methoxyphenol residues were connected to βCD using linkers with two different lengths, giving derivatives CD_1 and CD_2. Synthesized derivatives were characterized by 1H NMR, 13C NMR spectroscopies and mass spectrometry. The results confirmed expected structure of obtained derivatives and revealed high purity of the samples, which was also confirmed by electrochemical measurements, as described below.
For all substrates and synthesized βCD derivatives, electrochemical measurements in water and in organic aprotic solvent (DMSO) were carried out. Additionally, solubility tests of novel compounds were performed using spectrophotometry.
Solubility tests
The solubilities of βCD derivatives (CD_1 and CD_2) were determined using UV–Vis spectroscopy. The solubility of native βCD in pure water was equal to 18.5 mg/ml. For CD_1 and CD_2 the calculated values were 19.5 mg/ml and 17.9 mg/ml, respectively. The solubility of native βCD in water is the lowest among three most common cyclodextrins known as αCDs, βCDs and γCDs and is a result of odd number of glucopyranose units forming the ring. In the βCD, C2–OH group can form hydrogen bond with C3–OH group of adjacent glucopyranose unit. Formation of the complete belt of all hydrogen bonds makes the structure more rigid and probably leads to low solubility in water. Chemical modification disrupts hydrogen bonds belt and has influence on water solubility. The lipophilicity of the attached moiety is another feature that can be manipulated, and used to control water solubility of the modified CDs. In our present case, two linkers, with lipophilicity increasing with linker length, were used and respective CD derivatives were examined. The solubilities of the βCD derivatives in water decreased with the increase of chain length, hence lipophilicity of the linker connected to the CD ring increased. The solubilities of the derivatives presented here are significantly better than these obtained for similar βCD derivatives but with methoxyphenol group linked by sulfur or triazole group (0.28 mg/ml or 2.18 mg/ml, respectively) [8, 28]. This is due to more hydrophilic character of linkers presented in this paper. These kinds of linkers are of great value when designing novel water-soluble βCD derivatives.
Electrochemical measurements
Voltammetric curves (Fig. S5) for monoaldehyde and 4-(4methoxyphenoxy butyric) hydrazide recorded in an aprotic solvent (DMSO) have revealed the presence of irreversible oxidation peaks at similar potentials for both substrates that equaled to 1220 and 1225 mV, for monoaldehyde and the hydrazide, respectively. Electrochemical studies performed in the DMSO solution of CD_2 showed the presence of one irreversible oxidation peak with potential shifted towards more positive values (Fig. S5c). No signals referring to substrates used for the synthesis were visible, indicating high purity of the sample.
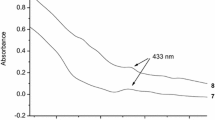
In aqueous solutions, no oxidation peaks were observed either for monoaldehyde or hydrazide. This is probably due to the pH-sensitivity of the functional group, reflected by the presence of the enol form in the case of monoaldehyde and poor solubility of hydrazide in water, respectively. On the other hand, voltammetry allows observing the hydrolysis reaction of CD_2, since in aqueous solution an oxidation peak at potential 1160 mV is developed, Fig. 1a. The current intensity is pH-dependent. In the pH range from 9 to 5, the decrease of the peak current was observed, until almost complete disappearance of the redox response, Fig. 1b. The dependence of peak current on pH demonstrates the hydrolysis of the hydrazone bond, which leads to the formation of primary substrates, that do not show electrochemical signals in aqueous solutions, Scheme 1. The separation of the side chain from the βCD core should decrease the complexing ability of the CD with aromatic molecules. In addition, the pH range of the cyclodextrin hydrolysis, which is similar to the pH of the tumor cells, gives the opportunity for hydrazone βCD derivatives to be used in selective delivery of anticancer drugs.
In order to confirm pH-sensitivity of the hydrazone derivatives of βCD, square wave voltammetry was employed to evaluate the stability constants of the CD_2–DOX complex at physiological pH (pH 7.4) and the pH of cancer cells (pH 5.5).
Upon addition of CD_2 to the DOX solution, the voltammetric peak currents decreased due to the smaller diffusion coefficient of the CD_2–DOX complex compared with that of the free guest, Fig. 2. The formation constants of the CD_2–DOX complex were calculated using Eq. 1. The dependencies of I2obs versus (I2DOX − I2obs)/[CD] for CD_2 at pH 7.4 and pH 5.5 obtained using SWV method are shown in Fig. 3a, b, respectively.
Osa dependencies (Eq. 1) for DOX in the presence of CD_2 at pH 7.4 (a) and 5.5 (b) recorded by SWV method in Britton–Robinson buffer
The values of the formation constants for the CD_2–DOX complex determined by SWV are: 5.24 ± 0.46 × 104 M−1 and 1.67 ± 0.30 × 104 M−1, for pH 7.4 and pH 5.5, respectively.
At physiological pH, the stability of the complex is slightly bigger than at pH 5.5. As we showed recently, the primary effect of β-CD–DOX complexes are strong proton-acceptor π–π interactions between the aromatic phenyl rings of the CD side group (Scheme 1) and anthraquinone group of doxorubicin (Scheme 2) [8]. At neutral pH (7.4), the electron-poor anthraquinone group strongly interacts with the electron-rich phenyl substituent of β-CD. At lower pH, the hydrolysis process leads to separation of the phenyl substituent from the cyclodextrin and the β-CD–DOX complex is no longer supported by the π–π interactions. This results in a weakening of the complex and the release of doxorubicin.
Conclusions
In present work, methoxyphenol residue was linked to βCD ring by two types of linkers containing hydrazone unit in a way to construct pH-sensitive derivatives of cyclodextrin for specific capsulation of aromatic drug—doxorubicin. The separation of the side chain from the βCD core decreased the complexing ability of the CD with aromatic molecules. The correlation between water solubility and linker length was observed, namely the shorter the linker, the more soluble the cyclodextrin derivative. It should be pointed out, that the solubilities of the derivatives presented here are significantly better than these of similar βCD presented previously. The presence of hydrazone bond in the linker made the βCD derivative pH-sensitive. Additionally, the range of pH at which the hydrolysis of the cyclodextrin occurs is similar to the pH of the tumor cells. We have demonstrated also, that newly synthesized derivatives of β-CDs containing aromatic side arms connected by hydrazone linker form strong inclusion complexes with DOX. The stability constants of the complexes were found to depend on the pH of the solution. At pH 7.4, corresponding to the pH of body fluids, the stability constants were bigger than those at pH 5.5, which is characteristic for cancer cells. That makes the newly synthesized derivatives attractive for pharmaceutical and medical applications.
References
Ueno, A., Breslow, R.: Selective sulfonation of a secondary hydroxyl group of β-cyclodextrin. Tetrahedron Lett. 23, 3451–3454 (1982)
Tripodo, G., Wischke, C., Neffe, A.T., Lendlein, A.: Efficient synthesis of pure monotosylated betacyclodextrin and its dimers. Carbohydr. Res. 381, 59–63 (2013)
Yoon, J., Hong, S., Martin, K.A., Czarnik, A.W.: A general method for the synthesis of cyclodextrinyl aldehydes and carboxylic acids. J. Org. Chem. 60, 2792–2795 (1996)
Huff, J.B., Bieniarz, C.: Synthesis and reactivity of 6-β-cyclodextrin monoaldehyde: an electrophilic cyclodextrin for the derivatization of macromolecules under mild conditions. J. Org. Chem. 59, 7511–7516 (1994)
Cornwell, M.J., Huff, J.B., Bieniarz, C.: A one-step synthesis of cyclodextrin monoaldehydes. Tetrahedron Lett. 36, 8371–8374 (1995)
Dess, D.B., Martin, J.C.: Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 48, 4155–4156 (1983)
Dess, D.B., Martin, J.C.: A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species. J. Am. Chem. 113, 7277–7287 (1991)
Swiech, O., Mieczkowska, A., Chmurski, K., Bilewicz, R.: Intermolecular interactions between doxorubicin and β-cyclodextrin 4-methoxyphenol conjugates. J. Phys. Chem. B 116, 1765–1771 (2012)
Swiech, O., Majdecki, M., Dembinski, A., Krzak, A., Stepkowski, T., Wojciuk, G., Kruszewski, M., Bilewicz, R.: Competition between self-inclusion and drug binding explains the pH dependence of the cyclodextrin drug carrier—molecular modeling and electrochemistry studies. Nanoscale 8, 16733–16742 (2016)
Krohn, K., Eds: Molecular mechanisms of anthracycline activity. In: Anthrcycline Chemistry and Biology, Mode of Action, Clinical Aspects and New Drugs, pp. 5–11, Springer, Berlin (2008)
Chaires, J.B., Herrera, J.E., Waring, M.J.: Preferential binding of daunomycin to 5′TACG and 5′TAGC sequences revealed by footprinting titration experiments. Biochemistry 29, 6145–6153 (1990)
Quigley, G.J., Wang, A.H., Ughetto, G., van der Marel, G., van Boom, J.H., Rich, A.: Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc. Natl. Acad. Sci. USA 77, 7204–7208 (1980)
Takemura, G., Fujiwara, H.: Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 49, 330–352 (2007)
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., Gianni, L.: Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 56, 185–229 (2004)
Myers, C.E., McGuire, W., Young, R.: Adriamycin: amelioration of toxicity by alpha-tocopherol. Cancer Treat. Rep. 60, 961–962 (1976)
Bekers, O., Kettenes, J.J., Van Helden, S.P., Seijkens, D., Beijnen, J.H., Bulti, A., Underberg, W.J.: Inclusion complex formation of anthracycline antibiotics with cyclodextrins; a proton nuclear magnetic resonance and molecular modelling study. J. Incl. Phenom. Mol. Recogn. Chem. 11, 185–193 (1991)
Husain, N., Ndou, T.T., De La Peña, A.M., Warner, I.M.: Complexation of doxorubicin with β and γ-cyclodextrins. Appl. Spectrosc. 46, 652–658 (1992)
Frömming, K.-H., Szejtli, J.: Cyclodextrins in Pharmacy. Kluwer Acad. Publ., Dordrecht (1994)
Duchêne, D.: New Trends in Cyclodextrins and Derivatives. Editions de Sante, Paris (1991)
Szejtli, J.: Cyclodextrin Technology. Kluwer Acad. Publ., Dordrecht (1988)
Duchêne, D.: Cyclodextrins and their Industrial Uses. Editions de Sante´, Paris (1987)
Loftsson, T.: The effects of cyclodextrins on the chemical stability of drugs in aqueous solutions. Drug Stab. 1, 22–33 (1995)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85, 1017–1025 (1996)
Brewster, M.E., Loftsson, T., Estes, K.S., Lin, J.L., Friđriksdóttir, H.: Effects of various cyclodextrins on solution stability and dissolution rate of doxorubicin hydrochloride. Int. J. Pharm. 79, 289–299 (1992)
Al-Ghorbani, M., Vigneshwaran, V., Ranganatha, V.L., Prabhakar, B.T., Shaukath, A.K.: Synthesis of oxadiazole–morpholine derivatives and manifestation of therepressed CD31 Microvessel Density (MVD) as tumoral angiogenic parameters in Dalton’s Lymphoma. Bioorg. Chem. 60, 136–146 (2015)
Mora, L., Chumbimuni-Torres, K.Y., Clawson, C., Hernandez, L., Zhang, L., Wang, J.: Real-time electrochemical monitoring of drug release from therapeutic nanoparticles. J. Controlled Release 140, 69; (2009)
Osa, T., Matsue, T., Fujihira, T.: Heterocycles 6, 1833–1839 (1977)
Swiech, O., Dutkiewicz, P., Wojciuk, K., Chmurski, K., Kruszewski, M., Bilewicz, R.: Cyclodextrin derivatives conjugated with aromatic moieties as pH-responsive drug carriers for anthracycline. J. Phys. Chem. B 117, 13444–13450 (2013)
Acknowledgements
The project was financed by the National Science Centre conferred on the basis of the decision number DEC-2011/01/N/ST5/05550.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Majdecki, M., Krzak, A., Żelechowska, K. et al. Monosubstituted hydrazone β-cyclodextrin derivatives for pH-sensitive complex formation with aromatic drugs. J Incl Phenom Macrocycl Chem 93, 77–83 (2019). https://doi.org/10.1007/s10847-018-0841-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0841-x