Abstract
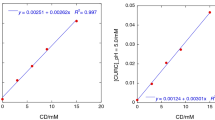
Cyclodextrin (CD) complex stoichiometry and complexation constant with two symmetric curcuminoids and two unsymmetric curcuminoid-like compounds were investigated and compared by two independent methods, the phase-solubility method and ultraviolet-visible absorption spectroscopy (UV–Vis) titration. Two different methods were applied in an effort to increase the apparent intrinsic solubility of the compounds and make the investigation of stoichiometry and complexation constants possible. The intrinsic solubility could be determined for all four compounds in aqueous 10% (v/v) ethanolic solutions. Higher order complexation or solubilization through complex aggregation was observed for the symmetric molecules, while 1:1 complexation was observed for the unsymmetric molecules in the phase-solubility diagram. The UV–Vis investigation showed 1:1 complexation for all compounds, with some indication of higher order complexation for the symmetric molecules. Thus the stoichiometry found with the two methods correlated well for the unsymmetric, but not for the symmetric compounds where the phase-solubility investigations clearly indicated higher order complexation and possible aggregation of complexes. There was also a difference between the 1:1 complexation constants found with the two methods, especially for the compounds with low intrinsic solubility (i.e. the symmetric curcuminoids). However, they agree in the ranking of complexes according to the strength of the association. The 1:2 complexation constant observed with the phase-solubility method was more than 100 times the complexation constant found with the UV–Vis method, which explains why solubility is poorly predicted from the UV–Vis data. This discrepancy may be explained by solubilization by aggregation of complexes or some phenomena other than inclusion complexation.





Similar content being viewed by others
References
Supardjan, A.M.: Chemical content of turmeric curcumin and its derivatives. Majalah Farmasi Indones. 12(3), 115–119 (2001)
Lauro, G.J., Francis, F.J.: Natural Food Colorants: Science and Technology. Marcel Dekker, Chicago (2000)
Tønnesen, H.H., Arrieta, A.F., Lerner, D.: Studies on curcumin and curcuminoids. Part XXIV. Characterization of the spectroscopic properties of the naturally occurring curcuminoids and selected derivatives. Pharmazie 50(10), 689–693 (1995)
Tønnesen, H.H., Grislingaas, A.L., Karlsen, J.: Studies on curcumin and curcuminoids. Part 19. Evaluation of thin-layer chromatography for the quantitation of curcumin and curcuminoids. Z. Lebensm.-Unters. Forsch. 193(6), 548–550 (1991)
Banerji, A., Chakrabarti, J., Mitra, A., Chatterjee, A.: Effect of curcumin on gelatinase A (MMP-2) activity in B16F10 melanoma cells. Cancer Lett. (Amsterdam, Netherlands) 211(2), 235–242 (2004)
Syng-ai, C., Kumari, A.L., Khar, A.: Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol. Cancer Ther. 3(9), 1101–1108 (2004)
Adams, B.K., Ferstl, E.M., Davis, M.C., Herold, M., Kurtkaya, S., Camalier, R.F., Hollingshead, M.G., Kaur, G., Sausville, E.A., Rickles, F.R., Snyder, J.P., Liotta, D.C., Shoji, M.: Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem. 12(14), 3871–3883 (2004)
Duvoix, A., Blasius, R., Delhalle, S., Schnekenburger, M., Morceau, F., Henry, E., Dicato, M., Diederich, M.: Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 223(2), 181–190 (2005)
Sui, Z., Salto, R., Li, J., Craik, C., Ortiz de Montellano, P.R.: Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1(6), 415–422 (1993)
Mazumder, A., Raghavan, K., Weinstein, J., Kohn, K.W., Pommier, Y.: Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 49(8), 1165–1170 (1995)
Artico, M., Di Santo, R., Costi, R., Novellino, E., Greco, G., Massa, S., Tramontano, E., Marongiu, M.E., De Montis, A., La Colla, P.: Geometrically and conformationally restrained cinnamoyl compounds as inhibitors of HIV-1 integrase: synthesis, biological evaluation, and molecular modeling. J. Med. Chem. 41(21), 3948–3960 (1998)
Vajragupta, O., Boonchoong, P., Morris, G.M., Olson, A.J.: Active site binding modes of curcumin in HIV-1 protease and integrase. Bioorg. Med. Chem. Lett. 15(14), 3364–3368 (2005)
Egan, M.E., Pearson, M., Weiner, S.A., Rajendran, V., Rubin, D., Gloeckner-Pagel, J., Canny, S., Du, K., Lukacs, G.L., Caplan, M.J.: Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science (Washington, DC, United States) 304(5670), 600–602 (2004)
Zeitlin, P.: Can curcumin cure cystic fibrosis? N. Engl. J. Med. 351(6), 606–608 (2004)
Gao, X., Kuo, J., Jiang, H., Deeb, D., Liu, Y., Divine, G., Chapman, R.A., Dulchavsky, S.A., Gautam, S.C.: Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem. Pharmacol. 68(1), 51–61 (2004)
Chueh, S.C.J., Lai, M.K., Liu, I.S., Teng, F.C., Chen, J.: Curcumin enhances the immunosuppressive activity of cyclosporine in rat cardiac allografts and in mixed lymphocyte reactions. Transplant. Proc. 35(4), 1603–1605 (2003)
Gomes Denise de Castro, F., Alegrio Leila, V., Leon Leonor, L., de Lima Marco Edilson, F.: Total synthesis and anti-leishmanial activity of some curcumin analogues. Arzneimittelforschung 52(9), 695–698 (2002)
Tønnesen, H.H., de Vries, H., Karlsen, J., Beijersbergen van Henegouwen, G.: Studies on curcumin and curcuminoids. IX: Investigation of the photobiological activity of curcumin using bacterial indicator systems. J. Pharm. Sci. 76(5), 371–373 (1987)
Anand, P., Kunnumakkara, A.B., Newman, R.A., Aggarwal, B.B.: Bioavailability of curcumin: problems and promises. Mol. Pharm. 4(6), 807–818 (2007)
Tønnesen, H.H., Masson, M., Loftsson, T.: Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm. 244(1–2), 127–135 (2002)
Tønnesen, H.H.: Solubility and stability of curcumin in solutions containing alginate and other viscosity modifying macromolecules. Studies of curcumin and curcuminoids. XXX. Pharmazie 61(8), 696–700 (2006)
Tønnesen, H.H., Karlsen, J.: Studies on curcumin and curcuminoids. V. Alkaline degradation of curcumin. Z. Lebensm.-Unters.-Forsch. 180(2), 132–134 (1985)
Tønnesen, H.H., Karlsen, J., van Henegouwen, G.B.: Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Z. Lebensm.-Unters.-Forsch. 183(2), 116–122 (1986)
Tønnesen, H.H.: Studies of curcumin and curcuminoids, XXVIII. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Pharmazie 57(12), 820–824 (2002)
Loftsson, T., Magnusdottir, A., Masson, M., Sigurjonsdottir, J.F.: Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 91(11), 2307–2316 (2002)
Loftsson, T., Hreinsdottir, D., Masson, M.: Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 302(1–2), 18–28 (2005)
Loftsson, T., Masson, M., Brewster, M.E.: Self-association of cyclodextrins and cyclodextrin complexes. J. Pharm. Sci. 93(5), 1091–1099 (2004)
Loftsson, T., Duchene, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329(1–2), 1–11 (2007)
Duan, M.S., Zhao, N., Oessurardottir, I.B., Thorsteinsson, T., Loftsson, T.: Cyclodextrin solubilization of the antibacterial agents triclosan and triclocarban: formation of aggregates and higher-order complexes. Int. J. Pharm. 297(1–2), 213–222 (2005)
Tomren, M.A., Masson, M., Loftsson, T., Tønnesen, H.H.: Studies on curcumin and curcuminoids. XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrins. Int. J. Pharm. 338(1–2), 27–34 (2007)
Szente, L., Mikuni, K., Hashimoto, H., Szejtli, J.: Stabilization and solubilization of lipophilic natural colorants with cyclodextrins. J. Inclusion Phenom Mol. Recognit. Chem. 32(1), 81–89 (1998)
Baglole, K.N., Boland, P.G., Wagner, B.D.: Fluorescense enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J. Photochem. Photobiol. A 173, 230–237 (2005)
Swaroop, S., Mishra, B., Priyadarsini, K.I.: Studies on b-cyclodextrin inclusion complex of curcumin. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 77(3), 205–211 (2007)
Tang, B., Ma, L., Wang, H.Y., Zhang, G.Y.: Study on the supramolecular interaction of curcumin and beta-cyclodextrin by spectrophotometry and its analytical application. J. Agric. Food Chem. 50, 1355–1361 (2002)
Qi, A.D., Li, L., Liu, Y.: The binding ability and inclusion complexation behavior of curcumin with natural alpha-, beta-, gamma-cyclodextrins and organoselenium-bridged bis (beta-cyclodextrin)s. J. Chin. Pharm. Sci. 12(1), 15–20 (2003)
Tønnesen, H.H., Karlsen, J., Adhikary, S.R., Pandey, R.: Studies on curcumin and curcuminoids. Part 17. Variation in the content of curcuminoids in Curcuma longa from Nepal during one season. Z. Lebensm.-Unters. Forsch. 189(2), 116–118 (1989)
Hegge, A.B., Màsson, M., Kristensen, S., and Tønnsen, H.H.: Investigation of curcumin-cyclodextrin inclusion complexation in aqueous solutions containing various alcoholic co-solvents and alginates using an UV–VIS titration method: studies on curcumin and curcuminoids XXXV. Pharmazie 64(6), 382–389 (2009)
Gibson, M.: Pharmaceutical Preformulation and Formulation A Practical guide from Candidate Drug Selection to Commercial Dosage Form. CRC Press LLC, Boca Raton (2004)
Pabon, H.J.J.: Synthesis of curcumin and related compounds. Recueil Des Travaux Chimiques Des Pays-Bas. J. R. Neth. Chem. Soc. 83(4), 379–386 (1964)
Babu, K.V.D., Rajasekharan, K.N.: Simplified condition for synthesis of curcumin I and other curcuminoids. Org. Prep. Proced. Int. 26(6), 674–677 (1994)
Venkateswarlu, S., Ramachandra, M.S., Subbaraju, G.V.: Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorg. Med. Chem. 13(23), 6374–6380 (2005)
Masuda, T., Matsumura, H., Oyama, Y., Takeda, Y., Jitoe, A., Kida, A., Hidaka, K.: Synthesis of (+–)-Cassumunins A and B, new curcuminoid antioxidants having protective activity of the living cell against oxidative damage. J. Nat. Prod. 61(5), 609–613 (1998)
Masuda, T., Hidaka, K., Shinohara, A., Maekawa, T., Takeda, Y., Yamaguchi, H.: Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J. Agric. Food Chem. 47(1), 71–77 (1999)
Quideau, S., Ralph, J.: Facile large-scale synthesis of coniferyl, sinapyl, and p-coumaryl alcohol. J. Agric. Food Chem. 40(7), 1108–1110 (1992)
Jovanovic, S.V., Steenken, S., Boone, C.W., Simic, M.G.: H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 121(41), 9677–9681 (1999)
Tønnesen, H.H., Karlsen, J.: Studies on curcumin and curcuminoids. VI. Kinetics of curcumin degradation in aqueous solution. Z. Lebensm.-Unters. -Forsch. 180(5), 402–404 (1985)
Bernabe-Pineda, M., Ramirez-Silva, M.T., Romero-Romo, M., Gonzalez-Vergara, E., Rojas-Hernandez, A.: Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 60A(5), 1091–1097 (2004)
Dietze, F., Arrieta, A.F., Zimmer, U.: pKa stability constants and UV/VIS spectral behavior of selected curcumin analogs. Pharmazie 52(4), 302–306 (1997)
Borsari, M., Ferrari, E., Grandi, R., Saladini, M.: Curcuminoids as potential new iron-chelating agents: spectroscopic, polarographic and potentiometric study on their Fe(III) complexing ability. Inorg. Chim. Acta 328, 61–68 (2002)
Wang, Y.-J., Pan, M.-H., Cheng, A.-L., Lin, L.-I., Ho, Y.-S., Hsieh, C.-Y., Lin, J.-K.: Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 15(12), 1867–1876 (1997)
Strickley, R.G.: Solubilizing excipients in oral and injectable formulations. Pharm. Res. 21(2), 201–230 (2004)
FDA. Inactive Ingredient Guide. http://www.fda.gov/cder/drug/iig/inact1.pdf (2008). Accessed 15 Dec 2008
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. (Washington, D C) 97(5), 1325–1357 (1997)
Mrozek, J., Guzow, K., Szabelski, M., Karolczak, J., Wiczk, W.: Influence of methanol and cyclodextrin cavity size on stoichiometry and binding constant of 3-[2-(9-anthryl)benzoxazol-5-yl]-alanine. J. Photochem. Photobiol. A 153(1–3), 121–128 (2002)
Pitha, J., Hoshino, T.: Effects of ethanol on formation of inclusion complexes of hydroxypropyl cyclodextrins with testosterone or with methyl orange. Int. J. Pharm. 80(2–3), 243–251 (1992)
Màsson, M., Sigurdardòttir, B.V., Matthìasson, K., Loftsson, T.: Investigation of drug -cyclodextrin complexes by a phase-distribution method: some theoretical and practical considerations. Chem. Pharm. Bull. 53(8), 958–964 (2005)
Connors, K.A.: Solubility measurement. In: Connors, K.A. (ed.) Binding Constants: The measurements of molecular complex stability, pp. 261–281. John Wiley and sons, Madison (1987)
Bender, M.L., Komiyama, M.: Cyclodextrin Chemistry. Springer-Verlag, Berlin; New York (1978)
Connors, K.A.: Determination of Stoichiometry. In: Connors, K.A. (ed.) Binding Constants: The measurement of molecular complex stability, pp. 24–28. John Wiley and Sons, Madison (1987)
Nelson, G., Patonay, G., Warner, I.M.: The utility of time-resolved emission spectroscopy in the study of cyclodextrin-pyrene inclusion complexes. Talanta 36(1–2), 199–203 (1989)
Connors, K.A.: Optical absorption spectroscopy. In: Connors, K.A. (ed.) Binding Constants: The Measurement of Molecular Complex Stability, pp. 141–188. John Wiley and Sons, Madison (1987)
Acknowledgements
The authors thank Ragnhild Haugse for the phase-solubility diagrams of curcumin and bisdemethoxycurcumin in CD formulations without ethanol, Hoai T.N. Aas from the Department of Pharmaceutics, University of Oslo, for the solubility determination of bisdemethoxycurcumin in ethanol and phase-solubility diagram of diketone in HPγCD and 10% (v/v) ethanol, and Tove Larsen, also from the Department of Pharmaceutics, University of Oslo, for the assistance with the calorimetric measurements. We thank the University of Iceland Research fund for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R., Tønnesen, H.H., Vogensen, S.B. et al. Studies of curcumin and curcuminoids. XXXVI. The stoichiometry and complexation constants of cyclodextrin complexes as determined by the phase-solubility method and UV–Vis titration. J Incl Phenom Macrocycl Chem 66, 335–348 (2010). https://doi.org/10.1007/s10847-009-9651-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9651-5