Abstract
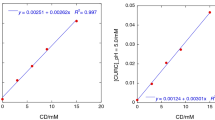
In the present study investigated the effect of curcumin (CUR), beta (β) and gamma (γ) cyclodextrin (CD) complexes on its solubility, stability, antioxidating activity and bioavailability. CUR the active principle of turmeric is a natural antioxidant agent with potent anti-inflammatory activity along with chemotherapeutic and chemopreventive properties. Poor solubility and poor oral bioavailability are the main reasons which preclude CUR use in therapy. Curcumin/CD complex systems were prepared and characterized by FT-IR, UV-Vis and 1HNMR spectroscopies. The content, solubility, dissolution, and stability of the complexes were evaluated and compared with curcumin and their physical mixture. The phase solubility analysis indicated that the solubility of CUR was increased in the presence of CDs and revealed an A(L)-type diagram, suggesting the formation of a 1:1 inclusion complex. The estimated apparent stability constant (K1:1), according to the Higuchi and Connors method, is 1.87 × 105 M−1 and 5.99 × 105 M−1 for CUR/β-CD and CUR/γ-CD respectively. The results of this study confirm the formation of inclusion complexes in solution and suggest that the complexes formation between CUR and CDs could improve the bioavailability of the drug due to the enhancing absorption expected from increased drug solubility. Furthermore, the antioxidant activities of CUR and CDs inclusion complexes were determined by the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) method. The experimental results confirmed the forming of CUR complexes with CDs also these indicated that the CUR/CDs inclusion complexes were the most reactive than its free form into antioxidant activity.


















Similar content being viewed by others
References
Helena, D.: Cyclodextrins and their Complexes: 1Molecules with Holes – Cyclodextrins, Wiley-VCH. Verlag GmbH. & Co. KGaA, Weinheim. ISBN: 3–527–31280-3 2006
Ramnik S, Nitin B, Jyotsana M, Hiremath SN (2010) Characterization of Cyclodextrin inclusion complexes – a review. J Pharm Sci Tech 2(3):171–183
Thorsteinn L, Marma S, Marcuse B (2004) Self Association of Cyclodextrins and Cyclodextrin Complexes. J Pharm Sci 93:1091–1099
Higuchi T, Connors KA (1965) Phase-solubility techniques. Adv Anal Chem Instrum 4:117–212
Loftsson T, Jarho P, Masson M, Jarvinen T (2005) Cyclodextrins in drug delivery. Expert Opin Drug Deliv 2:335–351
Francisco, B.T., Pessine, A.C. and Guilherme, L.A.: Review: Cyclodextrin Inclusion Complexes Probed by NMR Techniques. J. Mag Res Spect, ISBN 978–953–51-0065-2, 237–264 2012
Mousavifard B, Zeidabadinejad L, Pourastarabadi S, Dehestani M (2015) Investigation of interaction of vanillin with Alpha, Beta and Gamma cyclodextrin as drug delivery carriers: brief report. Tehran Univ. Med. J. (TUMJ) 73(2):132–137
Dhanaraju MD, Senthilkumarun K, Baskaran T, Sreeramamoorthy M (1998) Enhancement of Bioavailabity of griseofulvine by its complexation with cyclodextrin. Drug dev & Ind Pharm 24:583–587
Snezana S, Ilic S, Vesna DN, Ljubisa BN, Aleksandar SZ, Agnes JK, Mirjana MP, Slobodan DP (2015) The improved photostability of naproxen in the inclusion complex with 2-hydroxypropyl-β-cyclodextrin. Hem Ind 69(4):361–370
Gharibnaseri N, Ashnagar A, Husseini F (2007) Study of the inclusion Complexation of Piroxicam -β- Cyclodextrin and determination of the stability constant (K) by UV-visible spectroscopy. Scientia Iranica 14:308–315
Imran S, Vishal G, Abhay J, Naveen G (2011) Preparation and characterisation of B cyclodextrin aspirin inclusion complex. J Pharm & Life Sci (IJPLS) 2:704–710
Magnusdottir A, Masson M, Loftsson T (2002) Molecular modeling of diflunisal and ibuprofen in 2-hydroxypropyl-β-cyclodextrin cavity in the gas phase. J Incl Phenom Macrocycl Chem 44:213–218
Zidane S, Maiza A, Bouleghlem H, Herizi W, Dahmani S (2016) Investigation of Cyclodextrin inclusion compounds using FT-IR, SEM and X-ray diffraction. Inter J Chem Eng Appl 7:182–185
Erem M, Amelie B, Meralo Z, Murat S, Dominique D, Hincal AA (2003) Direct formation of Nanospheres from Amphiphilic β–Cyclodextrin inclusion complexes. Pharm Res 20(1):117–125
Ficarra R, Tommasini S, Raneri D, Calabro ML, Dibella MR, Rustichelli C, Gamberini MC, Ficarra P (2002) Study of flavonoids/β-cyclodextrins inclusion complexes by NMR, FT-IR, DSC. X-ray investigation J Pharm Biomed Anal 29:1005–1014
Bani-Yaseen AD, Moala A (2014) Spectral, thermal, and molecular modeling studies on the encapsulation of selected sulfonamide drugs in β-cyclodextrin nano-cavity. Spect Acta Part A: Mol Bio Spect 131:424–431
Jahed V, Zarrabi A, Bordbar A, Hafezi MS (2014) NMR (1H, ROESY) spectroscopic and molecular modeling investigations of supramolecular complex of β-cyclodextrin and curcumin. Food Chem 165:241–246
Mangolim CS, Moriwaki C, Nogueira AC, Sato F, Baesso ML, Neto AM, Matioli G (2014) Curcumin–β-cyclodextrin inclusion complex: stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem 153:361–370
Fabriciada RF, Iara BV, Edgar LCR, Maria T, Salles T, Claudio OA, Fernanda PC, Fabiane CA, Maríliaoliveira FG (2012) Antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. LWT- Food Sci Tech:1–6
Sandipan C, Soumalee B, Ansuman L, Soumen B (2010) Inclusion of chrysin in β-cyclodextrin nanocavity and its effect on antioxidant potential of chrysin: a spectroscopic and molecular modeling approach. J Mol Struc 977:180–188
Lauro MR, Carbone C, Auditore R, Musumeci T, Santagati NA, Aquino RP, Puglisi G (2012) A new inclusion complex of amlodipine besylate and soluble β-cyclodextrin polymer: preparation, characterizationand dissolution profile. J. Incl. Phenom. Macrocycl. Chem. Dol. https://doi.org/10.1007/s10847-012-0168-y
El-Kosasy AM, Tawakkol SM, Ayad MF, Sheta AI (2015) New methods for amlodipine and valsartan native spectrofluorimetric determination, with factors optimization study. Talanta. https://doi.org/10.1016/j.talanta.2015.05.012
Jadwiga M, Agnieszka C, Beata C (2006) Inclusion complexes of Felodipine and amlodipine with methyl-β-cyclodextrin. J Inc Phen Mac Chem 54:17–21
Agnes K, Vesna N, Ljubisa N, Mihajlo S, Milorad C, Ljiljana S, Dusica I (2010) Inclusion complexes of amlodipine besylate and cyclodextrins. Cent Eur J Chem 8(4):834–841
Berwin Singh SV (2013) Water Soluble Complexes of Curcumin and Hydroxypropyl Cyclodextrins: Preparation and Characterization. S.C.T.I. Med Sci Tech T:1–61
Ma M, Sun T, Xing P, Li Z, Li S, Su J, Chua X, Hao A (2014) A supramolecular curcumin vesicle and its application in controlling curcumin release, Colloids and Surfaces A. Physicochemical and Engineering Aspects. https://doi.org/10.1016/j.colsurfa.2014.06.043
Moussa Z, Hmadeh M, Abiad MG, Dib OH, Patra D (2016) Encapsulation of curcumin in cyclodextrin-metal organic frameworks: dissociation of loaded CD-MOFs enhances stability of curcumin. Food Chem 212:485–494
Alizadeh N, Malakzadeh S (2018) Spectroscopic study and antioxidant activity of the inclusion complexes of cyclodextrins and amlodipine besylate drug. J Incl Phen Mac Chem 90:89–98
Laura S, Lucreţia U, Ionut L, Zoltan S, Adriana F, Claudiu S (2016) β-Cyclodextrin inclusion complexes of lisinopril and zofenopril physicochemical characterization and compatibility study of lisinopril-β-cyclodextrin with lactose. J Therm Anal Calorim 123:2377–2390
Sambasevam KP, Mohamad S, Sarih NM, Ismail NA (2013) Synthesis and characterization of the inclusion complex of β-cyclodextrin and Azomethine. Int J Mol Sci 14:3671–3682
Jinxia L, Huizhi Z, Yanyan Y, Shumao S (2016) Study of the inclusion complex and antioxidating activity of Wogonin with β-cyclodextrin and hydroxypropyl-cyclodextrin. J Incl Phenom Mac Chem 84:115–120
Aadinath,W., Anu, B., Anandharamakrishnan, C.: Synergistic radical scavenging potency of curcumin-in-β-cyclodextrin-innanomagnetoliposomes Materials Science & Engineering C, doi: https://doi.org/10.1016/j.msec.2016.03.095 2016
Liu, M., Dong, L., Chen, A., Zheng, Y., Sun, D., Wang, X., Wang, B.: Inclusion complexes of quercetin with three β-cyclodextrins derivatives at physiological pH: Spectroscopic study and antioxidant activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. https://doi.org/10.1016/j.saa.2013.07.008 2013
Krishna Mohan PR, Sreelakshmi G, Muraleedharan CV, Roy J (2012) Water soluble complexes of curcumin with cyclodextrins: characterization by FT-Raman spectroscopy. Vib Spectrosc 2089:1–8
Rachmawati H, Edityaningrum CA, Mauludin R (2013) Molecular inclusion complex of Curcumin–β-Cyclodextrin nanoparticle to enhance Curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech 14:1303–1312
Acknowledgments
We acknowledge the financial support from university of Guilan and university campus 2, university of Guilan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alizadeh, N., Malakzadeh, S. Changes in chemical stability and bioactivities of curcumin by forming inclusion complexes of beta- and Gama-cyclodextrins. J Polym Res 27, 42 (2020). https://doi.org/10.1007/s10965-019-1994-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1994-z