Abstract
Odonates are widely considered to be bioindicators of freshwater habitat quality. Somatochlora arctica (Corduliidae) is commonly found across Eurasia, predominantly in North and Central Europe, but in the UK, it has a restricted range and is listed as near threatened despite a large, potential habitat availability. Across their range, larvae are found in Sphagnum-filled bog pools near coniferous woodlands, but detailed data on their habitat requirements are limited and often overlooked in favour of adult surveys which inhibits conservation efforts. This study surveyed three areas across Scotland: Abernethy & Loch Garten National Nature Reserve (NNR), Beinn Eighe NNR and Flanders Moss NNR, to evaluate how different environmental factors per pool (e.g. water depth and chemistry, Sphagnum coverage, woodland distance, etc.) affect the presence of S. arctica larvae. We found a higher occurrence of S. arctica larvae when pools were located close to woodlands (i.e. 0–10 m), and had high coverage of Sphagnum (> 90%). Environmental variables in pools surveyed (i.e. conductivity, water depth, pH and water temperature) had no significant effects on S. arctica presence.
Implications for insect conservation: Our results highlight and discuss the importance of nearby woodlands and Sphagnum cover for S. arctica larvae. Future conservation projects should consider the proximity of woodland to current and restored S. arctica sites or promoting expansion of surrounding natural woodland patches to benefit S. arctica and potentially other odonates sharing similar ecological preferences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improving the conservation efforts of rare and threatened aquatic species has the potential to benefit the wider conservation of freshwater ecosystems (Caro, 2010). Identifying indicator or umbrella species and focussing efforts on their conservation, can lead to the improvement of wider ecosystem processes. Odonates (dragonflies) are considered effective bioindicators of aquatic systems as they are affected by, and respond, to changing water quality and surrounding land use impacts (Oertli, 2008). As a result, some odonate species are associated with specific habitat conditions and can, therefore, be used as bioindicators of habitat quality and restoration efforts (Catling 2005; Seidu et al. 2018). For example, the rare and threatened Alpine emerald dragonfly (Somatochlora alpestris Selys, 1840) is associated with specific ecological requirements in upland pools and peatlands within the subalpine region, therefore, changes in the presence and distribution of the species are an indicator of wider changes in subalpine quality, relating to climate change (Kašák et al. 2023). Odonates are also impacted by anthropogenic activities e.g. pressures from overgrazing around ponds, eutrophication from fertilizers or responding to climate change by shifting their range (Chovanec and Raab 1997; Seidu et al. 2018; Termaat et al. 2019). Odonates also influence the wider trophic structure of their ecosystems as both predators and prey in freshwaters (Petermann et al. 2015). As odonates occupy both aquatic and terrestrial habitats in their larval and adult phases respectively, they are the subject of many studies on their ecological value and conservation (Villalobos-Jimenez et al. 2016) at local and global scales (Dolný et al. 2021). As a result of their responses to changing habitat conditions (Buczyńska and Buczyński, 2019), odonates have been often utilised as useful indicators of habitat restoration (Hodkinson and Jackson 2005; Oertli 2008).
Peatlands are valued for their carbon sequestration capacity and widely restored to maximise this potential; however, they generally do not support a large proportion of aquatic biodiversity due to their specific conditions, i.e. nutrient-poor, anoxic and acidic waters (Keddy 2010). Though this may exclude generalist species, the specialised conditions in peatlands can provide habitats for specific taxonomic groups e.g. species of odonates such as the pigmy damselfly (Nehalennia speciosa Charpentier, 1840) (Batzer et al. 2016; Minayeva et al. 2017; Orioli et al. 2021). However, relying on specialised habitat requirements can put species at risk, for example, a study on the decline in odonate diversity in low-elevation mountain wetlands (including peatlands) in Italy, found that approximately 32.6% of the odonate species historically occupying the area had either been extirpated or strongly declined compared to previous years, in part due to land-use change, environmental pollution and anthropogenic-driven climate warming (Assandri 2021). Our understanding of how invertebrate indicators respond to changes in peatland quality is still limited (Assandri and Bazzi 2022).
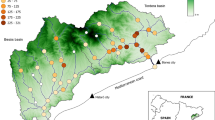
The Odonata family Corduliidae (Emeralds) is a relatively small family of odonates, with approximately 165 species (Smith and Patten 2020). Among these, the genus Somatochlora has several widespread species in both North America and Eurasia. However, despite the large geographical range over which this genus can be found, some species of Somatochlora prove challenging to locate due to their elusive flight behaviour and highly specialised habitat preferences, which results in many species occupying restricted habitat ranges (Smith and Patten 2020). This, coupled with invertebrates often being overlooked by conservation programmes, has resulted in a limited understanding of the use of peatland habitat by a species within this family; the Northern emerald dragonfly (Somatochlora arctica Zetterstedt, 1840). S. arctica is commonly found across Eurasia, predominantly North and Central Europe, but in the UK, populations of this species are listed as Near Threatened in the British Odonata Red List and restricted to isolated patches in central and north or northwest of Scotland, despite the existence of large, potential habitat availability (Batty 2014; Taylor et al. 2021) (Fig. 1).
Literature on habitat use by S. arctica in the UK is mostly observational, with a limited number of experimental studies. However, a study from the Netherlands observed S. arctica near densely filled Sphagnum pools in the vicinity of coniferous woodlands (Wildermuth 1986). This has been observed in several other populations across Eurasia, including the Carpathian Mountains and Scottish Highlands (Buczyński 1998; De Knijf et al. 2011; Batty 2014; Assandri and Bazzi 2022). Though habitat uses by S. arctica have been observed, they are rarely quantitatively tested. Furthermore, field studies on odonates tend to focus on adults, rather than the larval stage (Giugliano et al. 2012; da Silva-Méndez et al. 2022). This is a clear gap in the research, as the larval stage comprises the longest life stage for odonates (Stoks and Córdoba-Aguilar 2012) and represents a potentially more reliable source of data for field studies, as surveys are less reliant on optimal weather conditions for flight. Moreover, understanding how different habitat compositions affect the presence of larvae can help to provide greater insights into their conservation, as well as their potential use as bioindicators.
Given the paucity of knowledge on this species in the UK, especially regarding the environmental factors impacting the larval stage of the species, there is a need to obtain data regarding its presence and habitat use. These data could provide novel insights to better understand how the local habitat affects S. arctica presence at local and regional scales, with relevance for conservation across the species’ wider distribution. Thus, the objectives of this study were to: (i) evaluate the viability of larval sampling to determine the presence of S. arctica in Scotland, (ii) quantify the habitat preferences of S. arctica larvae, and (iii) use this information to inform future peatland and dragonfly conservation programmes. Based on previous studies and ad hoc observations, we hypothesise that S. arctica larvae will be predominantly found in pools with high Sphagnum coverage, located near coniferous woodlands with moderately acidic water conditions (pH = 4–6).
Materials and methods
Three areas across Scotland were surveyed: Flanders Moss NNR (56°08′04″N, 004°18′30″W), Abernethy and Loch Garten NNR (57°14′18″N, 003°42′35″W) and Beinn Eighe NNR (57°36′30″N, 005°18′44″W) (Fig. 2). Herein referred to as Flanders, Abernethy and Beinn Eighe. All three areas are managed by the Scottish Government’s Department for Natural Heritage, NatureScot. These areas were selected based on their recent records of S. arctica (British Dragonfly Society, 2019) and due to variations in their latitude and surrounding land use (Fig. 2). Flanders is an area of predominantly peatland in Central Scotland and is one of the largest (1073.33 ha) lowland ombrotrophic bogs in Britain (Cloy et al. 2005; NatureScot 2022c). Abernethy (12,755 ha), located in the Eastern highlands, is a mosaic of moorland, peatland and mixed woodland with large areas of Scots pine (Pinus sylvestris) (NatureScot 2022a). Beinn Eighe, located in North West Scotland, is smaller (480 ha) and is characterised by several scattered patches of ancient pine woodlands and fragmented peatland (NatureScot 2022b). The primary differences between the areas are the mean elevation (on average, 225 m at Abernethy, 82 m at Beinn Eighe NNR and 19 m at Flanders) and the woodland cover, which is highest at Abernethy (7678 ha of woodland, or 60% of the total reserve area) (JNCC, 2023) compared to Beinn Eighe NNR (223 ha, or 5% of the total reserve area) (NatureScot 2022a, b, c) and Flanders (247 ha, or 30% of the total reserve area) (JNCC, 2023).3
Each area was sampled at monthly intervals, from early May to the end of July 2022. Within each area, six plots were surveyed per visit (54 plots surveyed in the study overall). Plots were 20 × 20 m square quadrats and placed where S. arctica (larvae or adults) have previous been recorded. Each plot contained several Sphagnum-filled pools with all pools within each plot sampled, including pools located at the margin of a plot (Fig. 3). Studies have observed S. arctica larvae occurring at the margin of the bogs and fen habitats in dry areas with limited water and only moist sediment (Groenendijk and Bouwman 2010), but these habitats were rare within our survey areas and therefore not included.
Within each plot, pools were sampled using either a colander or a standard 1 mm mesh GB pond net to dislodge and sift vegetation (Fig. 4A and B). Debris in the colander or net were transferred to a white tray with any Odonata larvae sorted and placed into small containers for identification (Fig. 4C). Once sorted, all debris and invertebrates were returned to the pool they were collected from. The percentage of total Sphagnum coverage (%) was visually estimated per pool. The sampling effort per pool was an estimated 5–10 min per m2. Each bog pool was surveyed only once throughout the survey period to avoid pseudoreplication and to maximise the number of different pools surveyed under different habitat compositions.
There is evidence that S. arctica adults require nearby woodland for mating and resting, with adults observed circling pine and spruce trees and perching on branches during the mating season (Wildermuth 2003). Therefore, the distance to the nearest woodland was calculated in GIS (QGIS, version 3.30.1) using publicly available land use maps by determining the straight-line distance from the edges of the bog pools to the edges of the nearest woodland. Mature or semi-mature woodland sites with more than 10 trees were considered woodland. Single sapling trees were not considered woodland in this study. The type of woodland (coniferous, broadleaf, or mixed category) was visually assessed during surveys based on dominant tree species.
For each pool, pH, temperature (°C) and conductivity (µS/cm) were recorded using a Hanna Combo HI 98,129 probe for each visit. Both maximum and mean water depth (cm) were measured by using a 1 m stick; the mean was determined by measuring the water depth at approximately 5 m intervals around the edges of the pool as well as the middle sections (whenever possible).
Data exploration and analyses were carried out using R version 4.2.3 (R Core Team 2023). Summary tables for environmental data and S. arctica abundance per area were generated and tested using a Kruskal-Wallis test (as these data did were not normally distributed). Individual bog pools were considered as the sampling units in the model. As the number of S. arctica larvae per pool was generally low, these data were highly skewed and zero-inflated. Therefore, to assess the effect of environmental variables on the presence of S. arctica larvae per pool, we used a presence/absence (0 = no larvae found, 1 = larvae found) Binomial Regression Mixed Model (glmer, family = binomial). Survey area was used as a random slope in the model to account for geographic variation among sites.
All explanatory variables were checked for collinearity by Variance Inflation Factor (VIF). No collinearity was detected as all variables had VIF values around 1.5, therefore below the typically used threshold of 5. The categorical variable woodland type was removed from the final model as the majority of plots sampled were surrounded by coniferous woodland. No further variables were removed from the model as their removal or inclusion did not change the AIC score. The final model included seven continuous predictors: Sphagnum cover, presence/absence of other odonates (coded as a dummy variable, 0,1, so it could be included as an explanatory variable in the model) conductivity, water pH, water temperature, maximum pool depth, and distance to the nearest woodland (log-transformed). All predictors were scaled and standardised. The effect size of predictors was then visualised in ggplot via the Visreg package (Breheny and Burchett 2017).
Results
Table 1 shows variation in the local and landscape conditions per pool for the 3 survey areas. The main difference was the distance between the survey pools and the nearest woodland, which varied significantly between the three areas (p < 0.001). Plots at Abernethy had the lowest mean distances (7 m for all pools and 5 m for pools in which S. arctica were found) and sites at Flanders had the highest mean distances (159 m for all pools and 174 m for pools in which S. arctica was found). The nearby woodland type at Abernethy and Beinn Eighe was coniferous, generally Scots pine (Pinus sylvestris), while the closest woodlands to the survey plots at Flanders were mostly mixed woodlands or broadleaf tree species such as silver birch (Betula pendula) (Table 1).
Mean Sphagnum cover was significantly lower in pools at Beinn Eighe (p = 0.007); however, mean Sphagnum cover was very similar (76%) in the pools where S. arctica was found at Beinn Eighe and Abernethy. Conductivity also varied significantly (p < 0.001) between the three areas, with pools at Abernethy highest overall. The maximum water depth had similar ranges in the surveyed pools, except for Abernethy which were slightly (but not significantly, p = 0.2) shallower pools on average (Table 1). The mean water temperature and pH were similar among the 3 areas (Table 1).
Four species of odonate larvae were identified across all survey areas. In all three areas, we observed larvae of Libellula quadrimaculata Linnaeus 1758 (Four-Spotted chaser), Pyrrhosoma nymphula Sulzer 1776 (Large red damselfly), Aeshna juncea Linnaeus 1758 (Common hawker) and Somatochlora arctica (Northern emerald dragonfly) (Table 2). The highest number of S. arctica larvae per single pool was found at Abernethy (n = 6), however, but the highest total number of individuals was found at Beinn Eighe (Table 2). Few S. arctica larvae were found at Flanders.
S. arctica larvae were found at Abernethy and Beinn Eighe in all months surveyed, peaking in June (n = 9) and July (n = 8), respectively (Fig. 5). S. arctica larvae were found at Flanders in May and June, but the overall numbers were low.
The presence of S. arctica larvae was not significantly affected by conductivity, water depth, pH or water temperature (Table 3; Fig. 6). However, the presence of S. arctica was significantly greater (p = 0.011) at pools located close to woodland and with high Sphagnum coverage (p = 0.013) (Table 3; Fig. 6).
Discussion
In this study, we undertook monthly surveys of S. arctica larvae to determine potential drivers of their presence. Based on ad hoc and anecdotal evidence we expected the distance to the nearest woodland, Sphagnum cover and moderately acidic water conditions to influence the presence of S. arctica larvae (Batty et al. 2014) and our results broadly supported this. The distance between the pool and the nearest woodland had the strongest effect, a finding that largely aligns with previous observations (Wildermuth 1986, 2003; Groenendijk and Bouwman 2010; Batty 2014). Woodlands are essential for many odonates as they provide shelter, shade and food resources (Harms et al. 2014; O’Malley et al. 2020; Crabot et al. 2022) and may be linked to mating behaviour, as mating males and females of S. arctica have been previously observed hovering under the tree canopy (Wildermuth 2003). Similar observations of woodland use have been made for the Scottish populations of Brilliant Emeralds (Somatochlora metallica Vander Linden, 1825) which, in contrast to southern UK populations of the species and more widely in Europe, occupy wetland areas nearby woodlands, where they have been observed hovering near the tree canopy during mating (Cham 2022). As both S. arctica and S. metallica share a restricted habitat range in the north of Scotland this may reflect the limited quantity of pools with nearby suitable woodlands that provide essential resources for adult odonates. The majority of the pools surveyed in this study were found in areas near coniferous woodland (mainly comprised of Pinus sylvestris) which are the most common tree species in bog woodlands in Scotland (JNCC, 2023). However, there are records of S. arctica adults and larvae being found near broadleaf woodlands in the West of Scotland (Batty 2014), implying that the physical presence of a woodland, rather than the tree species composition per se, may be of importance to adults of S. arctica.
Although the importance of trees to adult odonates is noted in several studies (O’Malley et al. 2020; Crabot et al. 2022), the distance of woodlands from larval habitats has received less attention. We found that S. arctica larvae were more likely to be found in bog pools near woodlands. This suggests that the characteristics of the surrounding terrestrial habitat influence the adult selection of pools for oviposition. For odonates, the value of terrestrial habitats has historically been overlooked, relative to the importance of aquatic habitat quality (Dolny et al. 2019), despite current odonata distributions being derived predominantly from adult observations. However, considering both habitats as part of landscape-scale influences is becoming more common (Nagy et al. 2019; Dolny et al. 2021).
In general, local-scale factors, such as woodland type and distance, can help to predict the presence of the species within a limited area (e.g. predicting the optimal habitat), but landscape-scale analysis is required in order to assess the factors affecting species distributions at a national or international level. Potential landscape-scale factors impacting on the distribution of S. arctica include habitat fragmentation, as large areas of peatland in the UK have been lost or degraded due to commercial plantation of non-native trees, drainage (Wilson et al. 2005) and climate change, e.g. increased variability in weather patterns and overall temperature increases (Hickling, Hill and Thomas, 2005). Climate change will be a major driver of the distribution of S. arctica in the UK (Hickling, Hill and Thomas, 2005) and elsewhere, e.g. within its south-eastern European range, populations of S. arctica are predicted to display a shift northwards and upwards (De Knijf et al. 2011). Therefore, studies on current and future distributions of odonate species, need to incorporate both landscape and local factors e.g. climate prediction models, habitat suitability and species requirements, for both adult and larval stages.
Implications
Although a landscape-scale analysis is required to better understand the distribution of the species at a broad level, incorporating local-scale factors will provide conservation benefits. For example, by acknowledging the importance of nearby woodlands for S. arctica, conservation efforts can be focused within hotspots of the species, managing existing, and creating new habitats for both larvae and adults. Furthermore, the local-scale factors important to S. arctica are also an indication of wider habitat quality (i.e. high Sphagnum coverage), therefore, S. arctica may also be useful as a bioindicator of peatland habitat quality. However, further research is needed to separate the key drivers of S. arctica habitat preferences and use (e.g. preferred tree species, inter-specific competition, etc.).
Our results also highlight a gap between the conservation of S. arctica and current peatland restoration practices. Conventional peatland restoration is mostly carried out by removing trees and re-wetting through drainage ditch blocking (Lunt et al. 2010; Andersen et al. 2017). However, if S. arctica is to remain a part of bog and peatland biodiversity, these restoration approaches do not take into consideration their habitat requirements, which require nearby patches of woodland to complete their lifecycle. The use of small woodland corridors could be a solution, as these have been used successfully to support the conservation of other rare species (Haddad et al. 2015; Mendes et al. 2019; Kietzka et al. 2021). Albeit corridors would have to be placed considerately and likely be managed, as large tracts of woodland close to peatland may undermine restoration efforts for carbon sequestration and biodiversity (Minayeva et al. 2017; Szumińska et al. 2023). Allowing expansion of native coniferous woodland via regeneration surrounding peatland sites may be a more suitable resolution, combined with minor management. A further approach is to focus on the preservation and management of existing areas of peatland within or near the present range of S. arctica. For example, maintenance and creation of new pools that are a range of depths, limit high herbivore disturbance, whilst maintaining a high water table and high Sphagnum coverage. These conditions will be essential to provide resilience for peatland species during dry and warmer months that are expected to increase with climate change.
References
Andersen R, Farrell C, Graf M, Muller F, Calvar E, Frankard P, Caporn S, Anderson P (2017) An overview of the progress and challenges of peatland restoration in Western Europe. Restor Ecol 25(2):271–282
Assandri G (2021) Anthropogenic-driven transformations of dragonfly (Insecta: Odonata) communities of low elevation mountain wetlands during the last century. Insect Conserv Divers 14(1):26–39
Assandri G, Bazzi G (2022) Natural and anthropogenic determinants of peatland dragonfly assemblages: implications for management and conservation. Biodivers Conserv 31(2):703–722
Batty P (2014) Species review 8. J Br Dragonfly Soc, p.32
Batzer D, Wu H, Wheeler T, Eggert S (2016) Peatland invertebrates. Invertebrates in freshwater wetlands, pp.219–250
Breheny P, Burchett W (2017) Visualization of regression models using visreg. R J (Vol 9(2):56–71
Buczyńska E, Buczyński P (2019) Survival under anthropogenic impact: the response of dragonflies (Odonata), beetles (Coleoptera) and caddisflies (Trichoptera) to environmental disturbances in a two-way industrial canal system (central Poland). PeerJ 6:e6215
Buczyński P (1998) Somatochlora arctica (Zett.) In the Janowskie forests (Lasy Janowskie), SE Poland (Anisoptera: Corduliidae). Notulae Odonatologicae 5(1):8–9
Catling PM (2005) A potential for the use of dragonfly (Odonata) diversity as a bioindicator of the efficiency of sewage lagoons. Can Field-Naturalist 119(2):233–236
Cham S (2022) Brilliant emerald – a rare dragonfly with a bizarre distribution. Br Wildl 33(5):315–325
Chovanec A, Raab R (1997) Dragonflies(Insecta, Odonata) and the ecological status of newly created wetlands– examples for long-term bioindication programmes. Limnologica 27(3):381–392
Cloy JM, Farmer JG, Graham MC, MacKenzie AB, Cook GT (2005) A comparison of antimony and lead profiles over the past 2500 years in Flanders Moss ombrotrophic peat bog, Scotland. J Environ Monit 7(12):1137–1147
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/
Crabot J, Mauchamp A, Bergerot B, Bonis A, Gore O, Rossignol N, Paillisson JM (2022) How hydrology and landscape shape Odonata assemblages in marshlands crossed by ditches. Freshwater Biology
da Silva-Méndez G, Riso S, Lorenzo-Carballa MO, Cordero-Rivera A (2022) Sampling larvae, exuviae or adults of Odonata for ecological studies: a test of methods in permanent rivers in the Iberian Peninsula. Odonatologica 51(1–2):63–81
De Knijf G, Flenker U, Vanappelghem C, Manci CO, Kalkman VJ, Demolder H (2011) The status of two boreo-alpine species, Somatochlora alpestris and S. arctica, in Romania and their vulnerability to the impact of climate change (Odonata: Corduliidae). Int J Odonatol 14(2):111–126
Dolný A, Harabiš F, Mižičová H (2014) Home range, movement, and distribution patterns of the threatened dragonfly Sympetrum Depressiusculum (Odonata: Libellulidae): a thousand times greater territory to protect? PLoS ONE 9(7):e100408
Dolný A, Ožana S, Burda M, Harabiš F (2021) Effects of landscape patterns and their changes to species richness, species composition, and the conservation value of odonates (insecta). Insects 12(6):478
Giugliano L, Hardersen S, Santini G (2012) Odonata communities in retrodunal ponds: a comparison of sampling methods. Int J Odonatol 15(1):13–23
Groenendijk D, Bouwman H, J (2010) Occurrence and conservation of Somatochlora arctica in the Netherlands. Brachytron 12(1/2):18–24
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1(2):e1500052
Harms TM, Kinkead KE, Dinsmore SJ (2014) Evaluating the effects of landscape configuration on site occupancy and movement dynamics of odonates in Iowa. J Insect Conserv 18(3):307–315
Hickling R, Roy DB, Hill JK, Thomas CD (2005) A northward shift of range margins in British Odonata. Glob Change Biol 11(3):502–506
Hodkinson ID, Jackson JK (2005) Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ Manage 35(5):649–666
Joint Nature Conservation Committee (2023) Special Protected Areas – List of Sites: Abernethy Forest. https://jncc.gov.uk/our-work/list-of-spas/#scotland
Joint Nature Conservation Committee (2023) Bog Woodlands. H91D0. https://sac.jncc.gov.uk/habitat/H91D0/
Joint Nature Conservation Committee (2023) Flanders Mosses. https://sac.jncc.gov.uk/site/UK0012902
Kašák J, Holuša O, Mazalová M (2023) Artificial habitat–a chance for survival of a rare montane dragonfly (Odonata): case study on an alpine emerald (Somatochlora alpestris). J Insect Conserv 27(2):315–321
Keddy PA (2010) Wetland ecology: principles and conservation. Cambridge University Press
Kietzka GJ, Pryke JS, Gaigher R, Samways MJ (2021) Webs of well-designed conservation corridors maintain river ecosystem integrity and biodiversity in plantation mosaics. Biol Conserv 254:108965
Lunt P, Allott T, Anderson P, Buckler M, Coupar A, Jones P, Labadz J, Worrall P, Evans M (2010) Peatland restoration. Scientific Review commissioned by IUCN UK Peatland Programme Commission of Inquiry into Peatland Restoration, Edinburgh, UK
Mendes C, Dias E, Ponte M, Mendes A, Rochefort L (2019) The distribution and naturalness of peatland on Terceira Island (Azores): instruments to define priority areas for conservation and restoration. Mires and Peat, 24(35), p.16
Minayeva TY, Bragg O, Sirin AA (2017) Towards ecosystem-based restoration of peatland biodiversity. Mires Peat 19(1):1–36
Nagy HB, László Z, Szabó F, Szőcs L, Dévai G, Tóthmérész B (2019) Landscape-scale terrestrial factors are also vital in shaping Odonata assemblages of watercourses. Scientific reports, 9(1), p.18196
NatureScot (2022a) Abernethy National Nature reserve. https://www.nature.scot/enjoying-outdoors/visit-our-nature-reserves/abernethy-national-nature-reserve
NatureScot (2022b) Beinn Eighe and Loch Maree islands Nature Reserve. https://www.nature.scot/enjoying-outdoors/visit-our-nature-reserves/beinn-eighe-and-loch-maree-islands-national-nature-reserve
NatureScot (2022c) Flanders Moss Nature Reserve. https://www.nature.scot/enjoying-outdoors/scotlands-national-nature-reserves/flanders-moss-national-nature-reserve
O’Malley ZG, Compson ZG, Orlofske JM, Baird DJ, Curry RA, Monk WA (2020) Riparian and in-channel habitat properties linked to dragonfly emergence. Sci Rep 10(1):1–12
Oertli B (2008) The use of dragonflies in the assessment and monitoring of aquatic habitats. Dragonflies and damselflies: Model organisms for ecological and evolutionary research, pp.79–95
Orioli V, Gentili R, Bani L, Aguzzi S (2021) Microhabitat selection and population density of nehalennia speciosa charpentier, 1840 (Odonata: Coenagrionidae) in a peripheral microrefugium. Wetlands, 41(7), p.86
Petermann JS, Farjalla VF, Jocque M, Kratina P, MacDonald AAM, Marino NA, Srivastava DS (2015) Dominant predators mediate the impact of habitat size on trophic structure in bromeliad invertebrate communities. Ecology 96(2):428–439
Seidu I, Nsor CA, Danquah E, Lancaster L (2018) Odonata assemblages along an anthropogenic disturbance gradient in Ghana’s Eastern Region. Odonatologica
Smith BD, Patten MA (2020) Corduliidae: Emeralds. Dragonflies at a Biogeographical crossroads. CRC, pp 433–486
Stoks R, Córdoba-Aguilar A (2012) Evolutionary ecology of Odonata: a complex life cycle perspective. Ann Rev Entomol 57:249–265
Szumińska D, Czapiewski S, Sewerniak P (2023) Natural and anthropogenic factors influencing changes in peatland management in Poland. Reg Envriron Chang 23(1):1–20
Taylor P, Smallshire D, Parr A, Brooks S, Cham S, Colver E, Roy D (2021) State of Dragonflies 2021. British Dragonfly Society, UK
Termaat T, van Strien AJ, van Grunsven RH, De Knijf G, Bjelke U, Burbach K, Conze KJ, Goffart P, Hepper D, Kalkman VJ, Motte G (2019) Distribution trends of European dragonflies under climate change. Divers Distrib 25(6):936–950
Villalobos-Jimenez G, Dunn A, Hassall C (2016) Dragonflies and damselflies (Odonata) in urban ecosystems: a review. Eur J Entomol 113:217–232
Wildermuth H (1986) Zur Habitatwahl Und Zur Verbreitung Von Somatochlora arctica (Zetterstedt) in Der Schweiz (Anisoptera: corduliidae). Odonatologica 15(2):185–202
Wildermuth H (2003) Fortpflanzungsverhalten Von Somatochlora arctica (Zetterstedt)(Anisoptera: Corduliidae). Odonatologica 32(1):61–77
Wilson JD, Anderson R, Bailey S, Chetcuti J, Cowie NR, Hancock MH, Quine CP, Russell N, Stephen L, Thompson DB (2014) Modelling edge effects of mature forest plantations on peatland waders informs landscape-scale conservation. J Appl Ecol 51(1):204–213
Acknowledgements
This research was supported by the University of Stirling and we thank the landowners and reserve managers who allowed us access to their sites in this study. This manuscript was improved by comments provided by two anonymous reviewers.
Author information
Authors and Affiliations
Contributions
L.C. and A.L. conducted the field work and wrote the main manuscript text. L.C. prepared all figures and tables. P.B and D.M. provided help with dragonfly ID during field work and contributed to the writing of the sections on Northern Emerald distribution, behaviour and future implications for conservation. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cristofaro, L., Batty, P., Muir, D. et al. New insights on habitat use by larval Northern Emerald dragonflies (Somatochlora arctica). J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00599-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00599-8