Abstract
Most species co-evolve with their predators and develop strategies to avoid predation. This is not possible when a novel predator invades an environment. Native residents must quickly adapt to their new predator or face local extinction. Intense competition for mating opportunities exerts significant selective pressure that can drive the evolution of exaggerated structures across taxa. However, these elaborate traits can elevate the risk of predation for some organisms. In the present study, we observe the effect that rats have had on a population of endemic New Zealand stag beetles, Geodorcus helmsi. Rats in Rakiura | Stewart Island often prey on stag beetles, taking them to a sheltered area to eat them and discard any uneaten parts of the beetle, namely the head and mandibles. We compared the head size, mandible size and numbers of predated and non-predated male and female beetles in three sites in Rakiura | Stewart Island that differ in their abundance of mammalian predators. We found that the population demography and the size of the beetles differed significantly between sites. Additionally, we determined whether predated beetles were larger than non-predated beetles, across multiple years, regardless of site. We found that overall the predated specimens were larger than the non-predated beetles. The trends found here suggest that exaggeration of the male mandibles increases the predation risk of these iconic beetles, acting as a limit to mandible size.
Implications for insect conservation
Our results show for the first time the effect that novel predators can have on a population of animals with exaggerated sexually selected traits. The presence of novel predators can cause a shift in both population demography and trait distribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive mammalian predators are among the greatest drivers of biodiversity loss globally (Hilton and Cuthbert 2010; Szabo et al. 2012; Doherty et al. 2016). These species can lead to the decline or extinction of endemic fauna through a multitude of direct and indirect effects (Rayner et al. 2007; Doherty et al. 2015, 2016; Harper and Bunbury 2015; O’Donnell et al. 2017). Novel predators or parasites can exert strong selection on animal populations. For example, when the Australian field cricket, Teleogryllus oceanicus (Le Guillou, 1841), was introduced to the Hawaiian Islands, it encountered native parasitoid flies that were attracted to the courtship singing of the male crickets, a sexually selected trait. Resulting natural selection drove rapid evolution of non-calling males (Zuk et al. 2006). In a similar manner, invasive mammalian predators can impose strong selection on native species with sexually selected traits. Despite the diversity of animals that bear these features (Emlen 2008), this has not been well studied.
The animal kingdom displays considerable variation and diversity in sexually selected traits (Darwin 1872; Eberhard 1979; Emlen 2008). Many families are characterised by exaggerated sexually selected weapons or ornaments (e.g. mandibles on Lucanid beetles, eye stalks on Diopsid flies, forceps on earwigs (Dermaptera), claws on crabs (Brachyura); (Emlen 2008). Exaggerated traits that provide benefit in competition for mates require extra resources for their development and maintenance (Höglund et al. 1998; Allen and Levinton 2007; O’Brien et al. 2019). In holometabolous insects these traits grow to a fixed size during pupation (Emlen et al. 2006; Lavine et al. 2015), subject to their genetics and success of resource acquisition as larvae (Emlen et al. 2006; Lavine et al. 2015). Typically, females directly resource reproductive output in terms of egg number or quality (Koch and Meunier 2014), but males that develop exaggerated ornaments or weapons gain fitness benefits via sexual competition (Emlen 2008; Lavine et al. 2015). A number of studies have shown that having exaggerated traits may be detrimental to the survival of larger or more conspicuous males (Andersson 1994; Kotiaho et al. 1998; Zuk et al. 2006; Gwynne et al. 2007; Kojima et al. 2014; Ercit and Gwynne 2015; Goyens et al. 2015a, b; Okada et al. 2021; White et al. 2022). For instance, in the Japanese rhinoceros beetle, Trypoxylus dichrotomus (Linnaeus, 1771), males are preyed upon more often by mammalian and avian predators, particularly larger males with larger horns (Campanaro et al. 2011; Breitenmoser 2013; Kojima et al. 2014). In contrast, some studies do not find any correlation between predation risk and possession of exaggerated traits (Jennions et al. 2001; Worthington and Swallow 2010; Hongo 2010; Wehi et al. 2011; McCullough et al. 2012; McCullough and Emlen 2013; LeGrice et al. 2019; Yu et al. 2022). In these species, the costs associated with exaggerated traits may be mitigated over time as species co-evolve mechanical or behavioural adaptations to cope with these costs (Worthington and Swallow 2010; McCullough et al. 2012). For example, in the stalk eyed fly, Teleopsis dalmanni (Wiedemann, 1830), males are more likely than females to avoid predation by flying away, even in an enclosed space (Worthington and Swallow 2010). In these systems, a stable equilibrium is formed that reduces prey vulnerability to a potential predator (Pimenov et al. 2015). In systems exposed to invasive predators, this equilibrium may be disrupted as the behaviours these species use to mitigate predation may be less effective. This may result in strong selective pressure against the size of exaggerated traits (Zuk et al. 2006). Finally, it may also lead to a demographic shift as the sex bearing the exaggerated features may become rare in the environment. Surprisingly, no studies have explored how invasive predators might influence naïve species with exaggerated traits.
Geodorcus is a critically threatened genus of stag beetles consisting of 10 species (Holloway 2007) that are all endemic to Aotearoa | New Zealand. Like many stag beetles, Geodorcus has striking sexual dimorphism where the males have exaggerated mandibles and a larger overall body size compared to females. The longevity of beetles in this genus is unknown; however, it has been hypothesised that they may live up to 3 years as an adult (Holloway 2007). All species in this genus are flightless and slow-moving. Many of these species have ranges restricted to mountaintops or offshore islands (Holloway 2007). Native predators include avian fauna (thrushes (Turdus philomelos Brehm, 1831), mottled petrel (Pterodroma inexpectata (Forster, 1844)) and weka (Gallirallus australis (Sparrman, 1786))), or arachnids (Emberson 1975, pers. obs.). Introduced predators likely include pigs, hedgehogs and rats, but rodents are the main threat to these beetles.
The evolution of fauna and flora in New Zealand occurred largely in the absence of mammals (King 2019). Three species of rat arrived in New Zealand, aided by human sea travel: the Pacific rat (Rattus exulans (Peale, 1848)), the Norway rat (R. norvegicus (Berkenhout, 1769)), and the Ship rat (R. rattus Linnaeus, 1758) (King 2019; Russell et al. 2019). The Pacific rat came along with Polynesian explorers, in the thirteenth century, and the Norway rat and Ship rat arrived with European colonists in the nineteenth century (Wilmshurst et al. 2008). Rodents, and other mammalian predators, are implicated in the decline or extinction of many animals that were unfamiliar with these terrestrial, olfactory predators (Gibbs 2009; King 2019). Thus, Geodorcus do not have existing natural defences against mammal predators (Emberson 1975; Campbell et al. 1984; Gibbs 2009; Parkes et al. 2015). One species, Geodorcus ithaginis (Broun, 1893), is thought to be near extinction due primarily to rats (Sherley et al. 1994). Moreover, where rats occur on offshore islands, one widespread species, Geodorcus helmsi (Sharp, 1881) is absent, despite being on nearby predator-free islands (Bremner et al. 1984). It has been hypothesized that the presence of rats may affect the behaviour of G. helmsi, such that normally nocturnal beetles have become crepuscular or day active to coincide with periods of inactivity for rats (Johns 1982; Bremner et al. 1989). However, there have been no empirical studies looking at the direct impact rats have on populations of G. helmsi.
Many rodent species will scatter or hoard seeds in protected areas, also called husking stations, to secure adequate food supply, especially during adverse conditions (Barnett 1963; Keen-Rhinehart et al. 2010). Based on gut analysis, rats (Rattus spp. Linnaeus, 1758) eat a wide range of invertebrates and even birds, in addition to seeds (Clark 1982; Campbell et al. 1984; Taylor and Thomas 1993). Examination of Geodorcus remains indicate that rats will take the beetles and consume them in protected areas, similar to seed hoarding behaviour (Trewick and Morgan-Richards 2019). Over time, as they use that area more frequently, a cache of beetle remains will accrue (Fig. 1). Rats tend not to consume the anterior portion of the head and the mandibles (Trewick and Morgan-Richards 2019), which are heavily sclerotized chitin and contain minimal flesh. Thus, these rodent caches provide an opportunity to assess the effects of a novel predator on a population of native and naïve stag beetles.
Using G. helmsi, the only widespread species of Geodorcus (Holloway 2007), we aimed to test the predictions that (1) as larger beetles may be less efficient at evading rats or rats may prefer larger beetles as they represent a larger amount of food, larger males will be preyed upon more than smaller males, and (2) males will be more heavily affected by rats than female beetles as possessing exaggerated mandibles may decrease the mobility of male beetles. To our knowledge, this study is the first showing the effects of an invasive predator on an animal with exaggerated traits.
Methods.
Study sites
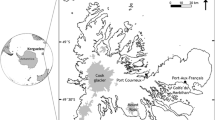
All fieldwork was conducted on Rakiura | Stewart Island, New Zealand. Rakiura has several invasive mammalian predators including hedgehogs (Erinaceus europaeus Linnaeus, 1758), feral cats (Felis catus Linnaeus, 1758), possums (Trichosurus vulpecula (Kerr, 1792) and rats (Rattus spp.) (www.predatorfreerakiura.org.nz/). Three species of rat are present (R. rattus Linnaeus, 1758, R. exulans, and R. norvegicus (Berkenhout, 1769)), but no mice. Fieldwork was conducted during three trips. The first collection (April 2017) was done by Steve Trewick, primarily at Garden Mound (Lat: − 46.868547, Long: 168.125493) and around Oban (Lat: − 46.898183, Long: 168.127999; Fig. 2). Trip 2 was conducted from 26 Jan 2021 to 1 Feb 2021 and samples were collected around Oban. The third field trip was conducted from 15 Feb 2022 to 11 Mar 2022. Three sites were visited: Ulva Island (Lat: − 46.926206, Long: 168.130816), Mamaku Point Conservation Reserve (Lat: − 46.872211, Long: 168.132721) and sites around Oban (Fig. 2). Due to scheduling conflict, different numbers of people participated in searching for beetles at each site: two people at Ulva Island, four people at Mamaku Point and two people in Oban.
Sampling locations on Rakiura Stewart Island. Closed points represent non-predated Geodorcus helmsi sampled. Darker open circles indicate approximate areas where caches were found, black points indicate specimens found in Mamaku Point, purple points indicate specimens found in Oban, yellow points indicate specimens found in Ulva Island. Map produced using leaflet R package. Map of New Zealand with bounding box to show where fieldwork was conducted in relation to the rest of New Zealand. Base map provided by Open Street Maps, CC-BY_SA
Oban is the main town on Rakiura | Stewart Island. It does not have any predator exclusion fencing, but there are trap lines that are regularly maintained. We searched approximately 32 hectares of this area. Ulva Island is a 267-ha island in the Patterson Inlet and has been a predator free sanctuary since 1997. The island is maintained by the Department of Conservation, with strict guidelines about protocol if rats are found. Mamaku Point, a 172-ha peninsula maintained by the Mamaku Point Conservation Trust, is an enclosed site with a predator free fence that was erected in 2000. Rats can invade by swimming from the nearby shores, but the population is kept low by an extensive trapping network.
Beetles were found during the day by looking under logs and at night by searching trees with headlamps. Each beetle was considered to be non-predated if they were not missing any body parts, whether alive or dead. Predated beetles were those with uneven and jagged tooth marks on decapitated heads (Fig. 3C, D). To find predated beetle heads, we searched through any crevices or concealed areas that a rat might use for shelter while eating. These concealed areas ranged from relatively small (between roots of a tree or in a crevice in tree bark) to large (under a log with a hollow space below it or in a tree hollow or hollow tree fern).
Measurements taken from predated and non-predated Geodorcus helmsi stag beetles. Measurement 1: mandible length, measurement 2: head width. Head width measure as width at eyes. Scale bar refers only to non-predated beetles. A Non-predated male beetle (N = 54 beetles), with arrow pointing to divot measured from on mandibles. B Non-predated female beetle (N = 287 beetles). C Predated male beetle head (N = 351 beetles), arrow pointing to tooth marks that have been left by rats, which continues around the posterior edge of head. D Predated female beetle head (N = 24 beetles). Images of non-predated G. helmsi obtained from Canterbury museum (Accession numbers: male: 2007.197.344, female: 2007.197.357
Measurements
When a live beetle was found, it was marked with paint using methods modified from (Painting and Holwell 2014), to ensure that beetles were not remeasured. Predated beetles were collected and measured in the lab. Mandibles were measured from the anterior edge of a divot on the lateral part of mandible to the mandible tip (Fig. 3). Only the left mandible was measured because the mandibles on G. helmsi are asymmetric with the right mandible being slight larger for many of the measurements (Thomas 2024). Head width was measured as the distance across the eyes. All beetles had their head width and mandible length measured using digital callipers (Fig. 3). These measurements were chosen as the head was often the only remaining intact body part of predated beetles (Fig. 3B). In this species, head width does have a positive allometric relationship with body length, but head width is also well correlated with body length (r = 0.92, p < 0.01, n = 134; Grey et al. 2024), and as such is used as a proxy for body length in the present study.
The allometric analyses included an expanded set of beetles from the April 2017 field trip, the 2020/2021 field trip, and the 2021/2022 field trip. To ensure that calliper measurements were consistent over time, measurements of head width and mandible length were redone on a subset of beetles (N = 32). We found that these calliper measurements were highly correlated for both head width (r = 0.91, p < 0.01) and mandible length (r = 0.91, p < 0.01).
Statistical analyses
All analyses were conducted using R version 4.0.3 (R Core Team 2022). All analyses were run with untransformed data.
Predator presence
All predator presence analyses were performed on only the 2021/2022 field data as other field trips did not sample from Ulva Island or Mamaku Point. Fisher’s exact test was used to determine whether there was any difference in number of beetles of each sex and their predation status. Additionally, Fisher’s exact test was used to identify any significant difference between predated and non-predated beetles in each area. Linear models were used to determine whether there was a difference between the mandible size and head width of predated and non-predated beetles between each site. We analysed predated beetles and non-predated beetles separately as no predated beetles were found on Ulva Island (0) and few non-predated beetles were found at Mamaku Point (5). Those sites were removed from the predated and non-predated analyses, respectively. The data were further subdivided by sex to determine if there was any difference in males or females between each site. Linear models were fitted to determine whether the presence of predators had any influence on mandible length and head width of predated and non-predated specimens.
Allometry
We performed an ANOVA, comparing mandible length and head width between predated and non-predated male beetles using pooled data from 2017, 2021 and 2022. As the beetles found on Ulva Island were significantly larger than those found in Oban (linear model: t = 3.31, p = 0.0018), we reran these analyses using only specimens from Oban. Additionally, to test whether populations with predators are investing proportionally less in mandible size, we performed an ANOVA on the ratio of head width and mandible length.
Results
Table 1 shows the number of predated and non-predated beetles found on the 2021/2022 field trip and a summary of the measurements collected for each group. There were more males than females in caches, but more non-predated females than males (Fisher’s test: p < 0.01, odds ratio: 0.018). The numbers of predated individuals were not distributed evenly among sites (Fisher’s test: p < 0.01), with predated individuals found at Oban and Mamaku Point, but none found at Ulva Island. Additionally, the numbers of non-predated beetles were not evenly distributed between each of the sites, with more non-predated individuals in Oban and Ulva Island than in Mamaku Point (Fig. 4).
Predated males in Mamaku Point had smaller heads (t = 5.03, p < 0.01) and mandibles (t = 5.03, p < 0.01) than those found in Oban (Fig.5 A, B). Non-predated male beetles from Oban had smaller heads (t = 2.553, p = 0.015) and mandibles (t = 2.161, p = 0.04) compared to beetles from Ulva Island (Fig.5 A, B), though when comparing both predated and non-predated Oban males to Ulva Island males, Oban tended to have larger males beetles overall (t = -2.667, p = 0.008). There was no difference in mandible length (t = 1.30, p = 0.21) and head width (t = − 0.893, p = 0.39) between sites for predated females (Fig.5 A, B). Similarly, there was no difference between sites for head width (t = − 1.53, p = 0.129) or mandible length (t = − 1.69, p = 0.09) for the non-predated females (Fig.5 A, B).
Allometry
Using the full dataset, which included data from 2017, 2021 and 2022, we obtained data for 375 predated beetles (24 female, 351 male; 342 complete heads, 33 mandibles only) and 341 non-predated beetles (287 female and 54 male). We found that non-predated beetle mandible lengths were smaller than those of the predated beetles (F1,395 = 33.10, p < 0.01) (Fig. 6A). Head width was larger for the predated beetles than for the non-predated beetles (F1,365 = 19.64, p < 0.01) (Fig. 6B). There were no differences for female mandible length (F1,307 = 1.07, p = 0.30) or female head width (F1,306 = 0.87, p = 0.35). Using only Oban specimens gave similar results; predated beetles were larger than non-predated beetles’ mandible length (F1,273 = 39.30, p < 0.01) and head width (F1,247 = 26.82, p < 0.01). There were still no differences for female mandible length (F1,295 = 0.97, p = 0.33) or head width (F1,294 = 1.38, p = 0.24). The ratio of mandible length to head width was smaller for non-predated males than it was for predated males (F1,359 = 36.82, p < 0.01). There was no difference in the ratio of mandible length to head width for females (F1, 306 = 0.295, p = 0.59).
Discussion
Our results indicate that in Geodorcus stag beetle populations with a significant number of predators, exaggerated mandibles may provide less benefit to competing over access to mating opportunities as males with exaggerated mandibles may evade predators less effectively than smaller males (Kojima et al. 2014). In this situation we see a reduction in mandible size in a population as the larger males are removed from the population. This can be seen as the direct impact of predation by rats, preferentially predating larger male stag beetles, either intentionally or unintentionally. The loss of these larger males may cause the average size of mandibles within these populations to decrease over time, assuming mandible size is a heritable trait (Kojima et al. 2014). This may in turn affect the stag beetle mating system as the larger beetles that may have been more suited to combat are no longer present. If these invasive mammalian predators continue to persist in these areas, then G. helmsi will be forced to either adapt strategies to avoid predation or suffer population decline. Unfortunately, our dataset does not allow us to assess year to year variation within the population, but future studies should test to determine if novel predators are causing a shift in the mandible and body size of these beetles.
One of the main costs associated with bearing sexually selected characters is that predators may “eavesdrop” on these signals and target these individuals, which has been observed in several insect taxa (Andersson 1994; Zuk et al. 2006; White et al. 2022). In some insect groups, the reproductive benefits of exaggerated traits remain despite trade-offs with survival as they have coevolved alongside their predators (Worthington and Swallow 2010; Hongo 2010; McCullough et al. 2012). However, when a novel predator appears, the anti-predatory behaviours that a species has evolved may not be as effective. This must exert selective pressure against any feature, such as large secondary sexual traits, that significantly reduce survival and thus reproductive fitness. We find no other studies examining the impact of introduced predators on insects with exaggerated morphologies, and this probably reflects the rapid, transitionary nature of novel species interactions. There are other species with exaggerated morphology in areas where mammalian predators have been introduced. These novel interactions have like caused many species to undergo rapid evolution to avoid predation (Carroll 2007; Gillis and Walsh 2017; Le Roux 2022). Further studies are required to determine the full effects that invasive mammalian predators have had on animals with exaggerated morphology.
In addition to a bias towards predation of larger Geodorcus male individuals, there also appears to be a bias towards preying upon male rather than female beetles, regardless of size. This may be due to males requiring more muscle in their head to operate larger mandibles and more thoracic muscle for locomotion of their bulkier bodies (O’Brien et al. 2019). Sexual signals can also be exploited by predators to locate prey (Promislow et al. 1992; Kotiaho 2001; Zuk et al. 2006; White et al. 2022) and as a result, sexual signalling can increase the risk of predation for the individual signalling (Gwynne 1987; Christe et al. 2006). In many species the signals are acoustic or colourful visuals and shifting the duration or pitch of when these signals are seen or heard can mitigate predation pressures (Kotiaho 2001). However, for animals with permanent sexual displays, like stag beetles, there is no way to reduce the amount of time a display is encountered. This could result in a novel predator rapidly shifting the prey population demography and mandible size distribution. As in other Lucanidae, smaller female G. helmsi are likely able to find refuges from predators much more efficiently than males, particularly large males (Thomaes et al. 2018). This bias in predation may increase the cost associated with possessing large mandibles, creating a trade off in terms of predation risk and competition for mates (Bierbach et al. 2011; Kojima et al. 2014; Torsekar et al. 2023).
On Ulva Island, the low numbers of beetles may be due to higher predation from natural predators of G. helmsi, like weka (G. australis), which are more abundant on Ulva Island because there are no rats on the island. To determine if this is a factor, we may be able to restrict a section of Ulva Island, or some other predator free location, and monitor the numbers of beetles found in each location.
Additionally, we found very few beetles in Mamaku Point Scientific Reserve. The habitat in this area had a similar habitat and flora to Ulva Island and Oban and we found some Geodorcus larvae under logs and stones. This indicates that Mamaku Point Scientific Reserve is suitable habitat for Geodorcus, so their scarcity may be due to rats entering the conservation area. However, a similar study on a reserve similar to Mamaku Point found that incursions were rare (Yarita et al. 2023). Once these rats are inside this area, they have a difficult time emigrating due to the fence restricting their movement. Considering the large number of predated beetles, there was once likely a much larger number of beetles in Mamaku Point. Additional trapping and rodent eradication measures should be taken to limit the number of rats in this environment.
The prevalence of rats in New Zealand has had a huge effect on the populations of native fauna and flora (Campbell et al. 1984; Clout 2001; Campbell and Atkinson 2002). Nationally, there has been increased effort to eradicate some non-native predators (possums (T. vulpecula), stoats (Mustela erminea Linnaeus, 1758), and rats (Rattus rattus, R. norvegicus, R. exulans) as part of the Predator Free 2050 (predatorfreenz.org) campaign. While control measures may temporarily decrease local predation pressure from rats, long term pest density persists (e.g. Yarita et al. 2023) and other mammalian predators including pigs and mice remain problematic for G. helmsi and other flightless insects. Previous studies have shown that pigs could have a dramatic effect on G. helmsi populations (Parkes et al. 2015). The effects of mice on G. helmsi are currently unknown, but mice densities will undoubtedly increase when rats are eradicated (Caut et al. 2007; Watts et al. 2022; Bertoia 2023).
The presence of predated beetles could also be a useful tool in determining whether rodents are present in an area. Identifying whether dead beetles were preyed upon by rats is relatively easy, as these beetles have obvious tooth marks left by a rodent attempting to chew through the head. While there are a number of other methods that are used for determining the presence of rats (e.g. baited tracking tunnels), looking for predated beetle heads may be useful on an initial survey of an area. Rat caches also represent areas that rats spend a large amount of time and would be areas to focus the placement of rodenticides (Campbell et al. 1984). Thus, G. helmsi may be a candidate bioindicator for the presence of rats in an area, though it is not known how long a predated beetle remains persist in the environment. Other species that may be good candidates as a bioindicator for the presence of rats include other large invertebrates that may be found in caches or husking stations, such as tree weta (Hemideina spp. Walker 1869), cockroach egg cases, and pupal cacoons Hymenoptera and Lepidoptera (Campbell et al. 1984).
It was not possible to determine the densities of rats from the locations sampled. As a result, we are not able to quantify predation levels. Future studies of predation intensity on G. helmsi or other ground dwelling invertebrates would ideally measure predator density/activity during the sampling period using tracking tunnels or cameras (Blackwell et al. 2002; Yiu et al. 2022). Additionally, hedgehogs are also predators of invertebrates (Jones et al. 2005; Jones and Norbury 2011) and may be exacerbating the effect that rats are having on the populations of stag beetles. However, little is known about the size and density of the populations of hedgehogs in Rakiura | Stewart Island (www.predatorfreerakiura.org.nz), or the impact that they are having on Geodorcus.
The findings of this study focus on the G. helmsi. However, these results may have significantly wider impacts. There are many species of flightless insect that follow a similar life history as G. helmsi in Aotearoa | New Zealand, particularly other beetle families (including Curculionidae, Tenebrionidae, Staphylinidae, etc.), wētā (H. spp.), cockroaches (Blattodea), earwigs (Dermaptera), and many other nocturnal insects (Campbell et al. 1984). These species may also be at risk of predation in areas with rats. Moreover, we have shown that animals with ornamentation face costs associated with predation. Similar species may be affected in the same way. Conservation management strategies and surveys should keep these factors in mind when assessing the threat classification for a species.
In conclusion, the extent of the effects that rats have had on native ecosystems of New Zealand has likely not yet been fully realized. The present study sheds additional light on how vulnerable G helmsi is to rats (Campbell et al. 1984; Bremner et al. 1984, 1989). Rats in Rakiura | Stewart Island have changed the population demography of this species to the point where male beetles, particularly large males, are relatively difficult to find compared to the females. Moreover, the presence of the rats also adds additional selective pressure acting against the exaggeration of the mandibles. These analyses were performed on the most widespread species of Geodorcus, but many of the species of Geodorcus have a threat classification associated with them (Sherley et al. 1994; Holloway 2007).
Data availability
Measurement data has been made publicly available at: https://doi.org/10.17605/OSF.IO/H3BTN.
References
Allen BJ, Levinton JS (2007) Costs of bearing a sexually selected ornamental weapon in a fiddler crab. Funct Ecol 21:. https://doi.org/10.1111/j.1365-2435.2006.01219.x
Andersson M (1994) Sexual Selection. Princeton University Press
Barnett SA (1963) Feeding Behaviour. In: The Rat. Routledge
Bertoia AJ (2023) Critters in the cold: understanding how large-bodied alpine invertebrates are influenced by introduced predators and climate change. University of Otago, Thesis
Bierbach D, Schulte M, Herrmann N et al (2011) Predator-induced changes of female mating preferences: innate and experiential effects. BMC Evol Biol 11:190. https://doi.org/10.1186/1471-2148-11-190
Blackwell GL, Potter MA, McLennan JA (2002) Rodent density indices from tracking tunnels, snap-traps and Fenn traps: do they tell the same story? N Z J Ecol 26:43–51
Breitenmoser S (2013) Etude de populations de Lucane cerf-volant Lucanus cervus ( L., 1758) (Coleoptera, Lucanidae) en zone périurbaine à Rolle (VD) de 2007 à 2012. Entomo Helvetica 6:49–61
Bremner AG, Barratt BIP, Butcher CF, Patterson GB (1989) The effects of mammalian predation on invertebrate behaviour in South West Fiordland. N Z Entomol 12:72–75. https://doi.org/10.1080/00779962.1989.9722570
Bremner AG, Butcher CF, Patterson GB (1984) The density of indigenous invertebrates on three islands in Breaksea Sound, Fiordland, in relation to the distribution of introduced mammals. J R Soc N Z 14:379–386. https://doi.org/10.1080/03036758.1984.10421738
Campanaro A, Toni I, Hardersen S, Grasso D (2011) Monitoring of lucanus cervus by means of remains of predation (Coleoptera: Lucanidae). Entomol Gen 33:79–89. https://doi.org/10.1127/entom.gen/33/2011/79
Campbell DJ, Atkinson IAE (2002) Depression of tree recruitment by the Pacific rat (Rattus exulans Peale) on New Zealand’s northern offshore islands. Biol Conserv 107:19–35. https://doi.org/10.1016/S0006-3207(02)00039-3
Campbell DJ, Moller H, Ramsay GW, Wait JC (1984) Observations on foods of kiore (Rattus exulans) found in husking stations on northern offshore Islands of New Zealand. N Z J Ecol 7:131–138
Carroll SP (2007) Natives adapting to invasive species: ecology, genes, and the sustainability of conservation. Ecol Res 22:892–901. https://doi.org/10.1007/s11284-007-0352-5
Caut S, Casanovas JG, Virgos E et al (2007) Rats dying for mice: Modelling the competitor release effect. Austral Ecol 32:858–868. https://doi.org/10.1111/j.1442-9993.2007.01770.x
Christe P, Keller L, Roulin A (2006) The predation cost of being a male: implications for sex-specific rates of ageing. Oikos 114:381–384. https://doi.org/10.1111/j.2006.0030-1299.15130.x
Clark D (1982) Foraging behavior of a vertebrate omnivore (Rattus Rattus): meal structure, sampling, and diet breadth. Ecology 63:763. https://doi.org/10.2307/1936797
Clout M (2001) Where protection is not enough: active conservation in New Zealand. Trends Ecol Evol 16:415–416. https://doi.org/10.1016/S0169-5347(01)02225-X
Darwin C (1872) The descent of man, and selection in relation to sex. D. Appleton
Doherty TS, Davis RA, van Etten EJB et al (2015) A continental-scale analysis of feral cat diet in Australia. J Biogeogr 42:964–975. https://doi.org/10.1111/jbi.12469
Doherty TS, Glen AS, Nimmo DG et al (2016) Invasive predators and global biodiversity loss. Proc Natl Acad Sci 113:11261–11265. https://doi.org/10.1073/pnas.1602480113
Eberhard WG (1979) The function of horns in Podischnus agenor (dynastinae) and other beetles. In: Blum MS, Blum NA (eds) Sexual selection and Reproductive Competition. Academic Press, New York, New York, pp 231–258
Emberson RM (1975) Bird predation on stag beetles. Entomol Soc N Z Sci Newsl 2:3
Emlen DJ (2008) The evolution of animal weapons. Annu Rev Ecol Evol Syst 39:387–413. https://doi.org/10.1146/annurev.ecolsys.39.110707.173502
Emlen DJ, Szafran Q, Corley LS, Dworkin I (2006) Insulin signaling and limb-patterning: candidate pathways for the origin and evolutionary diversification of beetle ‘horns.’ Heredity 97:179–191. https://doi.org/10.1038/sj.hdy.6800868
Ercit K, Gwynne DT (2015) Darwinian balancing selection: predation counters sexual selection in a wild insect. Evolution 69:419–430. https://doi.org/10.1111/evo.12579
Gibbs GW (2009) The end of an 80-million year experiment: a review of evidence describing the impact of introduced rodents on New Zealand’s ‘mammal-free’ invertebrate fauna. Biol Invasions 11:1587–1593. https://doi.org/10.1007/s10530-008-9408-x
Gillis MK, Walsh MR (2017) Rapid evolution mitigates the ecological consequences of an invasive species ( Bythotrephes longimanus ) in lakes in Wisconsin. Proc R Soc B Biol Sci 284:20170814. https://doi.org/10.1098/rspb.2017.0814
Goyens J, Dirckx J, Aerts P (2015a) Costly sexual dimorphism in Cyclommatus metallifer stag beetles. Funct Ecol 29:35–43. https://doi.org/10.1111/1365-2435.12294
Goyens J, Van Wassenbergh S, Dirckx J, Aerts P (2015) Cost of flight and the evolution of stag beetle weaponry. J R Soc Interface 12. https://doi.org/10.1098/rsif.2015.0222
Grey L, Holwell GI, Jandt JM, Johnson S (2024) Weapon allometry and shape variation in the Helm’s stag beetle (Geodorcus helmsi). Biol J Linn Soc blae024. https://doi.org/10.1093/biolinnean/blae024
Gwynne DT (1987) Sex-biased predation and the risky mate-locating behaviour of male tick-tock cicadas (Homoptera: Cicadidae). Anim Behav 35:571–576. https://doi.org/10.1016/S0003-3472(87)80283-X
Gwynne DT, Bussière LF, Ivy TM (2007) Female ornaments hinder escape from spider webs in a role-reversed swarming dance fly. Anim Behav 73:1077–1082. https://doi.org/10.1016/j.anbehav.2006.11.011
Harper GA, Bunbury N (2015) Invasive rats on tropical islands: their population biology and impacts on native species. Glob Ecol Conserv 3:607–627. https://doi.org/10.1016/j.gecco.2015.02.010
Hilton GM, Cuthbert RJ (2010) Review article: The catastrophic impact of invasive mammalian predators on birds of the UK Overseas Territories: a review and synthesis. Ibis 152:443–458. https://doi.org/10.1111/j.1474-919X.2010.01031.x
Höglund J, Sheldon BC, Hoglund J (1998) The cost of reproduction and sexual selection. Oikos 83:478. https://doi.org/10.2307/3546675
Holloway BA (2007) Lucanidae (Insecta: Coleoptera). Manaaki Whenua Press, Lincoln, Canterbury, NZ
Hongo Y (2010) Does flight ability differ among male morphs of the Japanese horned beetle trypoxylus dichotomus septentrionalis (Coleoptera Scarabaeidae)? Ethol Ecol Evol 22:271–279. https://doi.org/10.1080/03949370.2010.502322
Jennions MD, Moller AP, Petrie M (2001) Sexually selected traits and adult survival: a meta-analysis. Q Rev Biol 76:3–36. https://doi.org/10.1086/393743
Johns PM (1982) 1982: Insects collected on Secretary Island, November-December 1981. Unpubl Rep
Jones C, Moss K, Sanders M (2005) Diet of hedgehogs (Erinaceus europaeus) in the upper Waitaki Basin, New Zealand: Implications for conservation. N Z J Ecol 29:29–35
Jones C, Norbury G (2011) Feeding selectivity of introduced hedgehogs Erinaceus europaeus in a dryland habitat, South Island, New Zealand. Acta Theriol (warsz) 56:45–51. https://doi.org/10.1007/s13364-010-0009-6
Keen-Rhinehart E, Dailey MJ, Bartness T (2010) Physiological mechanisms for food-hoarding motivation in animals. Philos Trans R Soc B Biol Sci 365:961–975. https://doi.org/10.1098/rstb.2009.0225
King CM (2019) Invasive Predators in New Zealand: Disaster on Four Small Paws. Springer International Publishing, Cham
Koch LK, Meunier J (2014) Mother and offspring fitness in an insect with maternal care: phenotypic trade-offs between egg number, egg mass and egg care. BMC Evol Biol 14:125. https://doi.org/10.1186/1471-2148-14-125
Kojima W, Sugiura S, Makihara H et al (2014) Rhinoceros beetles suffer male-biased predation by mammalian and avian predators. Zoolog Sci 31:109–115. https://doi.org/10.2108/zsj.31.109
Kotiaho J, Alatalo RV, Mappes J et al (1998) Male mating success and risk of predation in a wolf spider: a balance between sexual and natural selection? J Anim Ecol 67:287–291. https://doi.org/10.1046/j.1365-2656.1998.00192.x
Kotiaho JS (2001) Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol Rev 76:365–376. https://doi.org/10.1017/S1464793101005711
Lavine L, Gotoh H, Brent CS et al (2015) Exaggerated trait growth in insects. Annu Rev Entomol 60:453–472. https://doi.org/10.1146/annurev-ento-010814-021045
Le Roux J (2022) Chapter 7 – Evolutionary impacts of invasive species on native species. In: Le Roux J (ed) The Evolutionary Ecology of Invasive Species. Academic Press, pp 135–158
LeGrice RJ, Tezanos-Pinto G, de Villemereuil P et al (2019) Directional selection on body size but no apparent survival cost to being large in wild New Zealand giraffe weevils. Evolution 73:762–776. https://doi.org/10.1111/evo.13698
McCullough EL, Emlen DJ (2013) Evaluating the costs of a sexually selected weapon: big horns at a small price. Anim Behav 86:977–985. https://doi.org/10.1016/j.anbehav.2013.08.017
McCullough EL, Weingarden PR, Emlen DJ (2012) Costs of elaborate weapons in a rhinoceros beetle: how difficult is it to fly with a big horn? Behav Ecol 23:1042–1048. https://doi.org/10.1093/beheco/ars069
O’Brien DM, Boisseau RP, Duell M et al (2019) Muscle mass drives cost in sexually selected arthropod weapons. Proc R Soc B Biol Sci 286:20191063. https://doi.org/10.1098/rspb.2019.1063
O’Donnell CFJ, Pryde MA, Van Dam-Bates P, Elliott GP (2017) Controlling invasive predators enhances the long-term survival of endangered New Zealand long-tailed bats (Chalinolobus tuberculatus): implications for conservation of bats on oceanic islands. Biol Conserv 214:156–167. https://doi.org/10.1016/j.biocon.2017.08.015
Okada K, Katsuki M, Sharma MD et al (2021) Natural selection increases female fitness by reversing the exaggeration of a male sexually selected trait. Nat Commun 12:3420. https://doi.org/10.1038/s41467-021-23804-7
Painting CJ, Holwell GI (2014) Observations on the ecology and behaviour of the New Zealand giraffe weevil (Lasiorhynchus barbicornis). N Z J Zool 41:147–153. https://doi.org/10.1080/03014223.2013.854816
Parkes JP, Easdale TA, Williamson WM, Forsyth DM (2015) Causes and consequences of ground disturbance by feral pigs (Sus scrofa) in a lowland New Zealand conifer–angiosperm forest. N Z J Ecol 39:34–42
Peltzer DA, Bellingham PJ, Dickie IA et al (2019) Scale and complexity implications of making New Zealand predator-free by 2050. J R Soc N Z 49:412–439. https://doi.org/10.1080/03036758.2019.1653940
Pimenov A, Kelly TC, Korobeinikov A et al (2015) Adaptive behaviour and multiple equilibrium states in a predator–prey model. Theor Popul Biol 101:24–30. https://doi.org/10.1016/j.tpb.2015.02.004
Promislow DEL, Montgomerie R, Martin TE (1992) Mortality costs of sexual dimorphism in birds. Proc R Soc Lond B Biol Sci 250:143–150. https://doi.org/10.1098/rspb.1992.0142
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Rayner MJ, Hauber ME, Imber MJ et al (2007) Spatial heterogeneity of mesopredator release within an oceanic island system. Proc Natl Acad Sci U S A 104:20862–20865. https://doi.org/10.1073/pnas.0707414105
Russell JC, Robins JH, Fewster RM (2019) Phylogeography of Invasive Rats in New Zealand. Front Ecol Evol 7. https://doi.org/10.3389/fevo.2019.00048
Sherley G, Green C, Owen K (1994) Distribution, conservation status and some features of the natural history of Dorcus stag beetles (Coleoptera: Lucanidae). Department of Conservation
Szabo JK, Khwaja N, Garnett ST, Butchart SHM (2012) Global patterns and drivers of avian extinctions at the species and subspecies level. PLoS ONE 7:e47080. https://doi.org/10.1371/journal.pone.0047080
Taylor RH, Thomas BW (1993) Rats eradicated from rugged breaksea island (170 HA), Fiordland, New Zealand. Biol Conserv 65:191–198. https://doi.org/10.1016/0006-3207(93)90052-3
Thomaes A, Dhont P, Dekeukeleire D, Vandekerkhove K (2018) Dispersal behaviour of female stag beetles (Lucanus cervus) in a mosaic landscape: when should I stay and where should I go. Insect Conserv Divers 11:523–533. https://doi.org/10.1111/icad.12325
Thomas B (2024) The ecology and behaviour of the endemic New Zealand stag beetle Geodorcus helmsi (Coleoptera: Lucanidae). University of Otago, Thesis
Torsekar VR, Zaguri M, Hawlena D (2023) Predation risk regulates prey assortative mating by reducing the expected reproductive value of mates. Ecology 104:e3869. https://doi.org/10.1002/ecy.3869
Trewick S, Morgan-Richards M (2019) Wild life New Zealand 2nd edition revised and extended. Hand-in-Hand Press in association with The New Zealand Centre for Planetary Ecology, Palmerston North
Watts C, Innes J, Wilson D et al (2022) Do mice matter? Impacts of house mice alone on invertebrates, seedlings and fungi at Sanctuary Mountain Maungatautari. N Z J Ecol 46(1):3472. https://doi.org/10.20417/nzjecol.46.22
Wehi PM, Nakagawa S, Trewick SA, Morgan-Richards M (2011) Does predation result in adult sex ratio skew in a sexually dimorphic insect genus? J Evol Biol 24:2321–2328. https://doi.org/10.1111/j.1420-9101.2011.02366.x
White TE, Latty T, Umbers KDL (2022) The exploitation of sexual signals by predators: a meta-analysis. Proc R Soc B Biol Sci 289:20220444. https://doi.org/10.1098/rspb.2022.0444
Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH (2008) Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc Natl Acad Sci 105:7676–7680. https://doi.org/10.1073/pnas.0801507105
Worthington AM, Swallow JG (2010) Gender differences in survival and antipredatory behavior in stalk-eyed flies. Behav Ecol 21:759–766. https://doi.org/10.1093/beheco/arq050
Yarita S, Morgan-Richards M, Trewick SA (2023) Genotypic detection of barriers to rat dispersal: rattus rattus behind a peninsula predator-proof fence. Biol Invasions 25:1723–1738. https://doi.org/10.1007/s10530-023-03004-8
Yiu SW, Gronwald M, Russell JC (2022) Reliable detection of low-density Pacific rats by using camera trapping. Wildl Res 50:398–411. https://doi.org/10.1071/WR22039
Yu G, Wong BH, Painting CJ, et al (2022) Males armed with big weapons win fights at limited cost in ant-mimicking jumping spiders. Curr Zool zoac101. https://doi.org/10.1093/cz/zoac101
Zuk M, Rotenberry JT, Tinghitella RM (2006) Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol Lett 2:521–524. https://doi.org/10.1098/rsbl.2006.0539
Acknowledgements
We thank Rose Borror, Sharn Milliken, Mateus Detoni and Melita Busch for their invaluable assistance with fieldwork. We thank Sheena Townsend for assistance with statical analyses. We would also like to thank the Department of Conservation and Mamaku Point Conservation Reserve for allowing us to sample beetles from Ulva Island and Mamaku Point, respectively. This study was done in accordance with the Department of Conservation permit 86347-FAU.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No authors have any financial or non-financial interests as it pertains to this study. This study was funded by a Doctoral Scholarship from the Department of Zoology at the University of Otago.
Author information
Authors and Affiliations
Contributions
L.G. collected data, analyzed data and prepared figures wrote main manuscript text as part of her Doctoral program. S.T. Revised text and provided initial data collection from 2017 and assisted with data analysis. S.J. Revised text and assisted in all areas as Supervisor during PhD program for L.G.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grey, L., Trewick, S.A. & Johnson, S.L. Introduced mammalian predators influence demography and trait variation of a New Zealand stag beetle. J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00593-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00593-0