Abstract
As evidence of global insect declines continues to mount, insect conservationists are becoming increasingly interested in modeling the demographic history of at-risk species from long-term survey data. However, certain entomological survey methods may be susceptible to temporal biases that will complicate these efforts. Entomological light traps, in particular, may catch fewer insects today than they once did due solely to increases in anthropogenic light pollution. Here we investigate this possibility by comparing the demographic histories of corn earworm moths (Helicoverpa zea) estimated from pairs of blacklight and pheromone traps monitored at the same farms. We find a stark decline in blacklight trap efficacy over 25 years of monitoring in Delaware, USA, mirrored over 10 years of monitoring in New Jersey, USA. While the precise causes of this decline remain a subject for discussion, the practical consequences are clear: insect conservationists cannot fully rely on long-term trends from entomological light traps.
Implications for insect conservation
H. zea populations appear to have remained largely stable over the past 25 years when assessed using pheromone trap data; when assessed using blacklight trap data, however, they appear to have declined precipitously. This disparity is consistent with a gradual loss in light trap efficacy due to concomitant increases in anthropogenic light pollution. Unfortunately, many nocturnal insects of conservation concern have historically been monitored via light traps alone. Going forward, insect conservationists should seek out alternative sources of monitoring data against which to calibrate estimates of demographic history obtained from light traps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect populations around the world appear to be in rapid decline (Dirzo et al. 2014; Leather 2018; Wagner et al. 2021a; Hordley et al. 2023; but see Wagner et al. 2021b; Yazdanian et al. 2023), with potentially disastrous consequences for the ecosystems of which they are part (Cardoso et al. 2020). In the face of a growing number of reports, conservationists are struggling to triage often limited resources to protect the most ecologically important and/or at-risk species (Harvey et al. 2020; Kawahara et al. 2021). Effective conservation action relies on a comprehensive understanding of the drivers of declines (Wagner 2019), which itself relies on accurate estimations of demographic histories (Thomas et al. 2019; but see Janzen and Hallwachs 2019). Many researchers have voiced concerns about the inherent difficulty of tracking insect populations over time, citing extreme interannual variation, limited historical data, and possible site selection bias (Didham et al. 2020). Sampling bias is another such complication: samples of insects caught in entomological traps are often used to approximate population numbers, but this approach can systematically underrepresent individuals that are — or have become — less likely to be trapped (Garamszegi et al. 2009; Martín-Vega and Baz 2013; Niemelä et al. 2015; Rhoades et al. 2017). In particular, sampling bias that varies over time has the potential to obfuscate genuine demographic shifts.
Entomological light traps use sources of artificial light, most commonly with a strong short-wavelength component (Jonason et al. 2014; White et al. 2016; Donners et al. 2018), to sample the abundance and diversity of insect communities. Light traps have several well-established sampling biases (McDermott and Mullens 2018): they are more likely to attract nocturnal insects than diurnal insects, macromoths than micromoths (Wölfling et al. 2016; see also Somers-Yeates et al. 2013; Merckx and Slade 2014), and males than females (Altermatt et al. 2009; Garris and Snyder 2010; Degen et al. 2016); they are also less effective under natural illumination from the full moon (Williams 1936; Taylor 1986; Yela and Holyoak 1997; Nowinszky and Puskás 2012). The potential for artificial illumination to interfere with light trap efficacy, however, while repeatedly suggested (Frank 2006; Eisenbeis 2006; McDermott and Mullens 2018; Didham et al. 2020; Grenis et al. 2023; see also Verheijen 1958) has yet to be conclusively tested (but see Conrad et al. 2006). Artificial light at night (ALAN) is a widespread environmental pollutant in both urban and rural habitats (Guetté et al. 2018; Cox et al. 2022; Fiorentin et al. 2022), one which has grown dramatically in intensity and extent over the past several decades (Sánchez de Miguel et al., 2021; Kyba et al. 2023). An increase in anthropogenic light pollution could systematically reduce the efficacy of light traps over time in one or more of the following ways.
First, an increased number of artificial light sources in the surrounding area may directly impact light trap catches through “light competition” (Conrad et al. 2006). When bespoke light traps were first adopted for systematic insect monitoring in the early 20th century (Williams 1936; Leather 2015), they were more likely to be among the only artificial light sources visible in the local environment at night. Today, an increasing number of road lights, house lights, lit windows, etc., may be dividing the attention of light-attracted insects (Didham et al. 2020), which would then become less likely to arrive at any one source in particular. Diffusion of artificial light through the atmosphere (often termed “skyglow”) is also increasing the overall level of night sky brightness, even in places relatively untouched by human development (Kyba et al. 2015). As a result, light traps may be losing visual contrast against the background and becoming more difficult for light-attracted insects to see. Both forms of light competition could in theory have reduced light trap catches in places where insect abundance and diversity have remained constant. This phenomenon should be more pronounced in more light-polluted habitats.
In contrast, rapid evolution of insect flight-to-light behavior has the potential to reduce light trap catches uniformly across space and time. While the various drivers of phototaxis in insects are yet to be fully understood (but see Hsiao 1973; Fabian et al., 2023), flight-to-light behavior is not generally thought to offer any fitness benefits to the individual. Rather, it is a maladaptive response to a novel stimulus without evolutionary analog, an oft-cited example of an evolutionary trap (Haynes and Robertson 2021). Light-attracted insects often suffer severe fitness consequences, dying from injury, exhaustion, or predation by bats, spiders, and other predators that have learned to exploit a captive food source (Owens et al. 2020; see also Boyes et al. 2021a, b); those that survive nonetheless lose vital opportunities to disperse, forage, mate, or oviposit (Owens et al. 2020). Over the past century, increasingly strong evolutionary pressure may have systematically selected for individuals that are inherently light-neutral or light-averse (e.g. Altermatt and Ebert 2016) or capable of learning to be so. In this way, rapid evolution also has the potential to have reduced light trap catches in places where insect populations are largely stable.
Historical light trap data alone cannot be used to investigate changes in light trap efficacy over time, as temporal biases are conflated with long-term changes in insect abundance (Conrad et al. 2006; Dively et al. 2018; Didham et al. 2020). Instead, what is needed are comparisons of light trap catch trends with baseline demographic histories, which might be inferred from samples obtained independently via trap methods less susceptible to anthropogenic interference. In this study, we leverage data from three targeted pest monitoring programs employing paired blacklight and pheromone traps at the same locations, monitored over the same period, to directly test for changes in light trap efficacy over time. Pheromone traps are baited with blends of synthetic female sex pheromones that attract reproductive males of a particular species (Hartstack et al. 1979) and are less likely than blacklight traps to be broadly influenced by light pollution or any other form of anthropogenic change. While blacklight and pheromone traps by default attract different amounts and types of insects (e.g. both sexes vs. only males), this difference should remain constant over time.
If the difference between blacklight and pheromone trap catches at the same location has changed over time, all else being equal, this would strongly suggest that one or both trap types have become better or worse at sampling the local insect population. Importantly, this finding would not significantly alter our broader understanding of insect declines. While many nocturnal insects, especially moths, have historically been monitored using entomological light traps alone (Fox et al. 2021; Wagner et al., 2021b), independent studies employing diverse sampling methods including malaise traps (Hallmann et al. 2017; Janzen and Hallwachs 2019), sticky traps (Lister and Garcia 2018), pitfall traps (Loboda et al. 2018), and transect counts (Forister et al. 2021) have all also detected declines in the abundance and diversity of insect communities. This looming ecological catastrophe makes it all the more crucial that we verify the accuracy of current estimates of insect demographic histories in order to better direct limited resources toward effective conservation action.
Methods
Helicoverpa zea (Lepidoptera: Noctuidae), commonly known as the corn earworm moth, is a highly prolific agricultural pest native to Central and North America. Unable to survive winter temperatures in the northern United States, H. zea overwinter as pupae in the southern United States (Lawton et al. 2022) and migrate north each spring via south winds (Westbrook and López 2010). Adult females oviposit on agricultural crops and their larvae can frequently be found eating through the silks of corn, fruit of tomatoes, or leaves of cotton (Fitt 1989). As the financial costs of infestation are high, farmers seek to control H. zea outbreaks via a combination of integrated pest management techniques, insecticides, and GMO crops. “Bt corn” engineered to express toxins from Bacillus thuringiensis soil bacteria had a strong initial impact on H. zea populations across the US (Farias et al. 2013; Dively et al. 2018) but the species has since rebounded following the rapid evolution of Bt resistance (Reay-Jones et al. 2020; Gassmann and Reisig 2023).
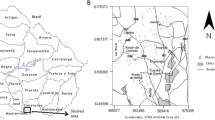
Due to their economic importance, H. zea populations have historically been and continue to be particularly well-monitored by agricultural entomologists. Cooperative extension programs across the United States employ networks of UV-rich light traps (hereafter referred to as blacklight traps) and/or pheromone traps to detect the onset of H. zea outbreaks. Trap catch data are shared directly with farmers to inform their decisions regarding the timing and location of pesticide application. While the majority of current H. zea monitoring networks across the US employ only a single trap type, those in Delaware, New Jersey, and Minnesota (Fig. 1 top) employ pairs of blacklight and pheromone traps placed at the same farms in close proximity.
The University of Delaware H. zea monitoring network has been running since 1998 and covers 11 farms. Blacklight traps at each farm capture light-attracted adults of both sexes using continuously operating T15 fluorescent bulbs (GE), replaced yearly, while nearby pheromone traps capture reproductive males attracted to synthetic female pheromone lures (Hercon Zealure), which are replaced approximately every two weeks. The number of moths captured in both of the traps at a farm are counted and the traps emptied every one to seven days in the summer months. The blacklight traps have largely remained at the same locations since the program began, while the pheromone traps move slightly each year following rotations in corn planting; nonetheless, the paired traps are never more than 0.53 km apart.
Locations of surveyed farms (top) and sample process for generating an abundance index from raw blacklight trap data obtained at a farm in Bridgeville, DE over summer 1998 (bottom). Map points correspond to the GPS locations of blacklight traps at monitored farms in Delaware (lime), Minnesota (blue), and New Jersey (green); Bridgeville, DE highlighted in lavender. Each collection event plotted below generated a nightly average number of H. zea moths caught (lavender points; moths caught divided by the number of nights traps were out between counts). Nightly averages were replicated across time (white points) to account for inconsistent intervals between collection events. From these, a single abundance index was calculated corresponding to the yearly average number of H. zea moths caught each night at a given farm. In this case, the abundance index equaled 2.2
The Rutgers University H. zea monitoring network in New Jersey has been covering seven farms consistently since 2013, again using pairs of continuously operating blacklight (F15T8/BL, Satco; previously purchased from Phillips and GE) and pheromone (Hercon Zealure) traps. Captured moths are counted every three to four days. While the blacklight traps are fully stationary, the pheromone traps have moved up to 2.4 km away to follow corn planting; distances vary across farms, with paired traps on average one kilometer apart. The University of Minnesota H. zea monitoring network has closely paired traps (F15T8/BL, GE; Hercon Zealure) in only one county, Dakota, where they have been operating since 2007. Captured moths are counted every one to eight days. Both the blacklight and pheromone traps have moved periodically over time, with a maximum of 0.53 km distance between them.
Comparison of estimated demographic histories
To investigate whether blacklight traps have lost efficacy relative to pheromone traps over time, we compared the demographic histories estimated from each trap type across each monitoring network statistically. We first divided total trap catches by the number of days traps were out prior to collection to estimate the nightly average number of moths caught per trap irrespective of differences in collection interval (e.g. every 3–4 days in Delaware vs. every 1–8 days in Minnesota; Fig. 1 bottom). We then filtered the data from each farm to only include days for which both blacklight and pheromone trap catch averages existed and only years that had 50 or more days of paired trap catch averages. All farms included in the final models described below had at least six sufficiently monitored years spanning a total period of eight years or more. Further details on the data gathered at each farm are provided in Table 1.
We combined filtered trap catch averages from the entire monitoring period of a given year, generally beginning in late May and ending in mid-September, to obtain a single estimated nightly count or “abundance index” for each unique combination of year, farm, and trap type, which was weighted to account for variation in sampling effort. We modeled the influence of year, trap type, and their interaction on this abundance index separately for each state. For the 25 years of monitoring data from Delaware, we were able to use a Gamma distributed generalized additive mixed model (GAMM) with a log link to capture long-term fluctuations. Short-term trends from New Jersey were more accurately captured via a generalized linear mixed model (GLMM; Gamma distribution, log link). To allow for a more direct comparison of results from these adjacent monitoring networks, we used only data obtained from the Delaware monitoring network over the same period (2013–2022) to build a matched GLMM. Models for New Jersey and Delaware (full and truncated) all included a random intercept of farm. Monitoring data from Minnesota came from only one farm and were therefore analyzed via a simple generalized linear model (GLM; Gamma distribution, log link) with the same suite of fixed effects.
All statistical analyses were performed in RStudio (R version 4.2.3, RStudio version 2023.03.0 + 386); models were built using functions from the packages lme4 (Bates et al. 2015) and mgcv (Wood 2011), and evaluated for fit and significance using functions from the packages car (Fox and Weisberg 2019) and emmeans (Lenth 2023).
Test for direct effects of environmental light
To investigate whether differences in the demographic histories estimated from each trap type are due to light competition, we regressed the difference between the paired abundance indices from each farm against environmental light levels estimated via satellite imagery. We used two methods (and three models) for this analysis: the first explored long-term trends on a year-by-year basis while the second compared average values at each farm from the past 10 years only.
Long-term trends in night sky brightness were obtained by harmonizing measurements from the stable DMSP/OLS NTL (1992–2013; Elvidge, 1997; Baugh et al. 2010) with those from the VIIRS day-night band (2013–2022; Elvidge et al. 2017) following Li et al. (2020). This harmonization method seeks to resolve imaging differences between the two satellites to create a geospatial dataset with greater temporal coverage, e.g. by decreasing the resolution of VIIRS images from 15 to 30 arc-seconds to match those taken by the DMSP. Harmonized nighttime light intensity is represented as a per-pixel digital number (DN) ranging from 0 to 63, with 0 indicating that no light was detected and 63 corresponding to full saturation of the DMSP sensor. As DN values below 7 tend to be unreliable (Li et al. 2020), we rounded these up to 7. DMSP data have many shortfalls (see Kyba 2016), but are the only satellite measure of global night sky brightness available prior to 2013.
To quantify the environmental light levels at each farm at night, we calculated the mean adjusted DN value in a one-kilometer buffer around the GPS coordinates of the blacklight traps at each farm, in each year, using Google Earth Engine (Gorelick et al. 2017). We then calculated a difference index, or a measure of the difference in the abundance indices of paired trap types, for each combination of year and farm by log-transforming the pheromone trap abundance index divided by the blacklight trap abundance index. A difference index greater than zero indicates that the pheromone trap caught more moths than its paired blacklight trap, and the degree of disparity scales with its magnitude. We modeled the influence of year and relative environmental light level (DN) on this difference index for all 20 farms across all three monitoring networks via a single GLMM containing random intercepts of year and farm.
In order to more uniformly compare results from the three independent monitoring networks, and to avoid relying on poorly calibrated DMSP data (Kyba 2016), we also modeled average values from the past 10 years at each farm. We used the method described above to estimate absolute environmental light levels (in nW/cm2/sr) in a one-kilometer buffer around each blacklight trap location over this time period, as calibrated satellite imagery is available for the full duration (2013–2022, VIIRS day-night band). We averaged these estimates and log-transformed the result to obtain a single measure of the absolute environmental light level for each farm. We also averaged the difference indices over the same 10-year time period to obtain a single average difference index for each farm. We modeled the influence of absolute environmental light level, monitoring network, and their interaction on this average difference index via a simple linear regression. A second version of this model instead using skyglow illuminance data (in mcd/m2) from Falchi et al. (2016)’s World Atlas of artificial night sky brightness is described in the Supplemental Text. All models were evaluated as described above.
Results
Across 25 years of monitoring in Delaware, on average blacklight traps caught approximately 30% as many H. zea moths as did pheromone traps (Gamma GAMM, log link; trap type: F = 198.54, df = 1.76, P < 0.0001). However, the disparity between trap catch averages scaled significantly with time (year by trap type interaction: F = 56.63, df = 7.86, P < 0.0001; Fig. 2 top). Over the first five years of monitoring, blacklight traps caught about half as many moths as did pheromone traps (blacklight trap abundance index: 3.63 ± 0.82; pheromone trap abundance index: 7.65 ± 0.75, mean ± SE in this and following reports; Fig. 3 left). Pheromone trap catch averages remained relatively constant over time (estimated population growth rate [λ] = 1.009 [0.993–1.025, 95% CI in this and following reports]) while those of blacklight traps declined precipitously (λ = 0.858 [0.843–0.873]), such that in the last five years blacklight traps caught only 4.6% as many moths as did pheromone traps (pheromone trap abundance index: 9.67 ± 0.58; blacklight trap abundance index: 0.45 ± 0.03; Fig. 3 right). As a result, the H. zea population in Delaware appeared to have declined by approximately 33% over the past 25 years (λ = 0.984 [0.969–1.000]; year: F = 59.58, df = 17.10, P < 0.0001) when modeled using data from both trap types.
Temporal comparison of yearly average H. zea moth catches collected from paired pheromone (orange) and blacklight (violet) traps belonging to monitoring networks in Delaware (top), New Jersey (bottom left), and Minnesota (bottom right). Data from Delaware have also been truncated (bottom center) to the range of years monitored in New Jersey to facilitate comparisons between these two adjacent monitoring networks. Points represent average moths caught per night at a given farm in a given year while lines and bands represent predicted mean and 95% confidence intervals of modeled trends, respectively. All y-axes are log-transformed. Blacklight traps in New Jersey and Delaware (both full and truncated) exhibit a statistically significant loss of efficacy relative to pheromone traps over time, while the blacklight trap in Minnesota does not
Nightly average H. zea moth catches in pheromone (orange) and blacklight (violet) traps throughout the summer across the entire Delaware monitoring network from the first five years of monitoring (left, 1998–2002) compared to the most recent five years of monitoring (right, 2018–2022). Five-year averages across all farms for each day of year are displayed as points with associated lines and bands representing mean and 95% confidence intervals of modeled trends, respectively. The inset plot shows the data from the most recent five years on a log scale
In New Jersey, the H. zea population appeared to increase approximately fivefold over a decade of monitoring (λ = 1.220 [1.122–1.326]; Gamma GLMM, log link; year: likelihood ratio χ2 = 32.68, df = 1, P < 0.0001) across trap types. On average, blacklight traps caught only 4.2% as many moths as did pheromone traps (trap type: LR χ2 = 520.76, df = 1, P < 0.0001), and this disparity also grew significantly over time as pheromone trap catch averages increased (λ = 1.230 [1.158–1.306] while blacklight trap catch averages remained constant (λ = 1.050 [0.985–1.120]; year by trap type interaction: LR χ2 = 11.90, df = 1, P = 0.0006; Fig. 2 bottom left). Over the same period, the H. zea population in Delaware followed a similar trajectory (λ = 1.131 [1.067–1.120]), again driven by an increase in pheromone trap catch averages (λ = 1.142 [1.104–1.183] relative to blacklight trap catch averages (λ = 1.031 [0.998–1.065]; Gamma GLMM, log link; year: LR χ2 = 43.31, df = 1, P < 0.0001; trap type: LR χ2 = 1699.10 df = 1, P < 0.0001; year by trap type interaction: LR χ2 = 17.83, df = 1, P < 0.0001; Fig. 2 bottom center).
In Minnesota, the H. zea population at Dakota declined across 16 years of monitoring (Gamma GLM, log link; year: LR χ2 = 4.70, df = 1, P = 0.0302). Their demographic history was broadly consistent regardless of whether it was modeled only using pheromone trap catch averages (λ = 0.947 [0.862–1.040]), only using blacklight trap catch averages (λ = 0.926 [0.840–1.020]), or by integrating data from both trap types (λ = 0.936 [0.875–1.001]). On average, blacklight traps caught 4.3% as many moths as did pheromone traps (trap type: LR χ2 = 81.50, df = 1, P < 0.0001) and there was no evidence that this disparity increased or decreased over time (year by trap type interaction: LR χ2 = 0.13, df = 1, P = 0.7166; Fig. 2 bottom right).
The environmental light level at each farm was not directly related to the difference between pheromone and blacklight trap catch averages, whether modeled using unique data from each year or average values from the past 10 years. Although the disparity between trap types increased over time across monitoring programs (Gaussian LMM; year: LR χ2 = 57.82, df = 1, P < 0.0001), the difference index at a given farm in a given year was independent of the relative environmental light level within a one-kilometer buffer around that farm (DN value: LR χ2 = 1.07, df = 1, P = 0.3009). Over the past 10 years, the average difference index at each monitored farm varied significantly across monitoring programs but was independent of the corresponding average absolute environmental light level (linear regression; monitoring network: F = 4.38, df = 2, P = 0.0334; light level: F = 2.61, df = 1, P = 0.1283). A positive relationship between these values at farms in Delaware (slope = 0.56 ± 0.43) was counterbalanced by a negative relationship at farms in New Jersey (slope = -0.49 ± 0.28; monitoring network by light level interaction: F = 18.95, df = 1, P = 0.0007; Fig. 4), meaning that more light polluted farms (as estimated by satellite imagery) tended to have more disparate trap catch averages in Delaware but the reverse was true in New Jersey. For an analysis using skyglow estimates from Falchi et al. (2016)’s World Atlas of artificial night sky brightness in lieu of raw values from satellite imagery (following Simons et al. 2020), see Supplemental Text.
Average difference between pheromone and blacklight catches over the past 10 years at each farm plotted against the corresponding average absolute environmental light level. Average difference indices have been log-transformed, with values greater than zero indicating that pheromone traps caught more moths than blacklight traps. Average light level has been calculated from the log-transformed surface radiance (in nW/cm2/sr) within a one-kilometer buffer around the GPS location of the blacklight trap at each farm, as estimated from satellite imagery (VIIRS day-night band). Mean and 95% confidence intervals of significant relationships between these values in Delaware (lime) and New Jersey (teal) are displayed as dotted lines and solid bands, respectively
Discussion
Entomological light traps have been used for over a century to survey insect abundance and diversity (Slingerland 1902) and feature prominently in several long-term insect monitoring networks (e.g. the Rothamsted Insect Survey, the Hungarian Forestry Light Trap Network, the Finnish National Moth Monitoring Scheme). Although the nocturnal light environment has changed dramatically over the past several decades, population models built from light trap data frequently assume that light traps of the same make and model sample the same proportion of their local insect community today as they did when first deployed. We tested this assumption by comparing the demographic histories of H. zea estimated from paired blacklight and pheromone traps at 19 farms belonging to three independent long-term monitoring networks. As pheromone traps should be relatively unaffected by most forms of anthropogenic change, we used them as a control against which to test for changes in blacklight trap efficacy. We found that catch averages from the two trap types diverged significantly over the last decade in the two most thoroughly sampled monitoring networks. In both cases, data from blacklight traps indicated lower H. zea population growth rates than did data from pheromone traps, consistent with a loss in blacklight trap efficacy over time. Although these data only represent one particularly well-monitored crop pest, our findings suggest that entomologists should view species demographic histories estimated from long-term light trap monitoring networks with greater skepticism.
Paired pheromone and blacklight traps monitored over 25 years in Delaware captured the same apparent fluctuations in the H. zea population from which they were sampling: growth between 2004 and 2011 followed by a steep decline lasting until 2015, perhaps due to the popularization of Bt corn, followed by recovery. Year over year, however, blacklight traps caught a progressively smaller proportion of the moths caught by their paired pheromone traps. Population models built only using data from blacklight traps or by integrating data from both trap types suggest that H. zea has declined, while a control model built only using data from pheromone traps finds (correctly, we posit) that the population has remained stable. The paired traps monitored over 10 years in New Jersey follow a similar pattern, especially when compared to the same period in Delaware, likely in part because the University of Delaware and Rutgers University monitoring networks are relatively geographically close (< 100 km average distance) and were sampling from a single highly mixed population (Seymour et al. 2016). The 10-year population growth rate estimated only using data from pheromone traps is positive, as H. zea is likely in recovery, but blacklight traps appear to be sampling a progressively smaller proportion of this growing population each year. The divergence between trap types is less extreme over this period as blacklight traps were already catching far fewer moths than were pheromone traps at the first year of sampling.
Because any genuine population fluctuation would be reflected equally in samples from both trap types, regardless of its root cause, our results provide strong evidence for a loss in blacklight trap efficacy over time. An alternative explanation would be that pheromone traps have become more attractive to moths over time, improving in sampling efficacy. This is unlikely to be the case, however, as the chemical composition of the artificial H. zea female pheromones used in these traps has remained constant and species-specific scent-based signals should be robust to most forms of anthropogenic noise (Endler 1992). Light-based signals, by contrast, are known to decline in attractiveness following increases in environmental light (Eisenbeis 2006; Owens et al. 2022). Recent growth in anthropogenic light pollution has been sufficiently extreme — in some places doubling night sky brightness within only the past decade (Kyba et al. 2023; see also Sánchez de Miguel et al., 2021) — to account for an extreme loss in blacklight trap efficacy. It is also worth noting that a decline in blacklight trap efficacy may have indirectly led to a small increase in pheromone trap efficacy, as male moths freed from blacklight traps would become available to nearby pheromone traps. Interestingly, blacklight trap catches in Delaware primarily diverged from pheromone trap catches between 2010 and 2014. Ultra-bright, blue-rich LED streetlights were rapidly becoming standard over this period (Zissis et al. 2021) and may have played some role in driving this trend; in Delaware, pilot programs for LED streetlight retrofits began as early as 2010 (Delmarva Power, 2010).
A loss in blacklight trap efficacy could be a direct result of increased light competition in the immediate area, whether that be from streetlights or other local sources of artificial light, or it could be an indirect result of behavioral plasticity and/or rapid evolution, both of which might affect the entire highly mixed population approximately equally. We used multiple geospatial approaches to investigate whether nearby environmental light levels drove the observed trends but found no overarching relationship between the difference between pheromone and blacklight trap catches and local light pollution levels, both of which varied widely across and within states. Previous attempts to correlate aerially-collected light pollution levels with moth blacklight trap catches have also failed to uncover any relationship (Conrad et al. 2006; White 2018). Given the breadth of evidence demonstrating that natural light from the full moon significantly reduces blacklight trap efficacy (Taylor 1986; Yela and Holyoak 1997; Nowinszky and Puskás 2012), the lack of correlation between artificial light and blacklight trap catch is curious. One potential explanation calls into question the current practice of measuring ecological light pollution (sensu Longcore and Rich 2004) from space.
Concerns have been raised about the use of satellite imagery for measuring terrestrial light levels, given the frequent disparity between these measurements and reports from citizen scientists on the ground (Kyba et al. 2023). The VIIRS day-night band cannot detect short wavelengths below 500 nm, where the majority of the spectral sensitivity of most moth species is concentrated (van der Kooi et al. 2021), and is also biased towards upwelling light (Kyba et al. 2022). These fundamental limitations have been worsened by the global transition to blue-rich LEDs in relatively well-shielded fixtures (Kyba et al. 2023), such that LED streetlight retrofits frequently result in noticeably brighter skyglow on the ground that is measured by the VIIRS day-night band as a decrease in surface radiance (Barentine et al. 2018; Hung et al. 2021). Light-attracted insects are disproportionately attracted to UV and blue light (van Langevelde et al. 2011; Donners et al. 2018; Deichmann et al. 2021), and habitats with a higher proportion of short-wavelength light pollution may therefore experience greater loss of blacklight trap efficacy. Unfortunately, the VIIRS day-night band is intrinsically incapable of capturing this relationship. A between-state difference in the adoption of blue-rich LEDs and/or shielding may explain why, averaged over the last decade, we see a positive correlation between absolute environmental light levels and blacklight vs. pheromone trap disparities at farms in Delaware but a negative correlation between the same variables at farms in New Jersey.
Behavioral plasticity and rapid evolution are two possible alternative explanations for a loss in blacklight trap efficacy that need not necessarily be linked to environmental light levels in the immediate area. H. zea moths travel hundreds of miles across the US each summer (Lawton et al. 2022). Over their 2–3 week lifespan (Armes et al. 1992), individuals have multiple opportunities to learn the costliness of light attraction, most of which would occur at areas far from where they are ultimately trapped or not trapped. Behavioral plasticity alone could explain the loss in blacklight trap efficacy, as opportunities for learning from encounters with artificial light at night have increased over time. However, rapid evolution may be a more convincing explanation given the short lifespan and fast generation time of this species. One common garden experiment uncovered consistent behavioral differences between urban and rural small ermine moths (Yponomeuta cagnagella), with urban individuals significantly less attracted to blacklight traps (Altermatt and Ebert 2016). Decades of selective pressure acting on 3–7 generations per year may have culminated in a broad-scale loss of flight-to-light behavior across the entire range of H. zea, which has much higher dispersal rates and many more generations per year than univoltine Y. cagnagella (Altermatt and Ebert 2016) as well as a demonstrated capacity for rapid evolution in response to strong selection (Reay-Jones et al. 2020). Evolutionary loss of flight-to-light behavior would not necessarily nullify the fitness costs of light pollution, as relevant adaptations may involve a decline in flight ability (Van de Schoot et al. 2024), decreased nocturnal activity, impaired visual sensitivity, or some other impediment to usual function.
In this study, we found a significant and consistent temporal bias in blacklight trap data, which caused resulting demographic models to incorrectly report dramatic declines in a prominent crop pest. From a management perspective, our results suggest that agricultural entomologists should prioritize other data sources (pheromone traps, bait traps, pitfall traps, etc.) when tracking pest outbreaks and formulating pesticide application recommendations, especially early in the season when numbers are low. Multiple redundant sources of monitoring data are even more valuable from a conservation perspective. Much of our earliest insect abundance and diversity data come from light traps; for many species, especially nocturnal moths, these data comprise the only historical point of comparison. In the absence of any independent, contemporaneous data source to compare against, insect conservationists should be aware that apparent population declines may result primarily or solely from declines in the attractiveness of the light traps themselves. Similar comparative studies in other species, where possible, could help uncover how widespread this phenomenon is and may even culminate in the creation of tools for time-calibrating light trap data. Such tools would allow us to more accurately weigh historical reports against the current reality, which is that an increasing number of artificial lights on earth each individually attract fewer and fewer insects.
Data availability
Data used in this study will be made publicly available online via Figshare upon publication.
References
Altermatt F, Ebert D (2016) Reduced flight-to-light behaviour of moth populations exposed to long-term urban light pollution. Biol Lett, 12(4)
Altermatt F, Baumeyer A, Ebert D (2009) Experimental evidence for male biased flight-to-light behavior in two moth species. Entomol Exp Appl 130(3):259–265
Armes NJ, Bond GS, Cooter RJ (1992) The laboratory culture and development of Helicoverpa armigera. Natural Resources Institute
Barentine JC, Walker CE, Kocifaj M, Kundracik F, Juan A, Kanemoto J, Monrad CK (2018) Skyglow changes over Tucson, Arizona, resulting from a municipal LED street lighting conversion. J Quant Spectrosc Radiative Transf 212:10–23
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear mixed-effects models using lme4. J Stat Softw 67:1–48
Baugh K, Elvidge C, Tilottama G, Ziskin D (2010) Development of a 2009 stable lights product using DMSP-OLS data. Proceedings of the Asia-Pacific Advanced Network, 30
Boyes DH, Evans DM, Fox R, Parsons MS, Pocock MJO (2021a) Is light pollution driving moth population declines? A review of causal mechanisms across the life cycle. Insect Conserv Divers 14(2):167–187
Boyes DH, Evans DM, Fox R, Parsons MS, Pocock MJO (2021b) Street lighting has detrimental impacts on local insect populations. Sci Adv 7(35):eabi8322
Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kwak ML, Mammola S, Ari Noriega J, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Settele J, Simaika JP, Stork NE, Suhling F, Vorster C, ..., Samways MJ (2020) Scientists’ warning to humanity on insect extinctions. Biol Conserv 242:108426
Conrad KF, Warren MS, Fox R, Parsons MS, Woiwod IP (2006) Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol Conserv 132(3):279–291
Cox DTC, Sánchez de Miguel A, Bennie J, Dzurjak SA, Gaston KJ (2022) Majority of artificially lit Earth surface associated with the non-urban population. Sci Total Environ 841:156782
de Sánchez A, Bennie J, Rosenfeld E, Dzurjak S, Gaston KJ (2021) First estimation of global trends in nocturnal power emissions reveals acceleration of light pollution. Remote Sens 13:16
Degen T, Mitesser O, Perkin EK, Weiß N-S, Oehlert M, Mattig E, Hölker F, Chapman J (2016) Street lighting: sex-independent impacts on moth movement. J Anim Ecol 85(5):1352–1360
Deichmann J, Gatty C, Navarro J, Alonso A, Linares-Palomino R, Longcore T (2021) Reducing the blue spectrum of artificial light at night minimises insect attraction in a tropical lowland forest. Insect Conserv Divers 14:247–259
Delmarva Power Launches LED Streetlight Pilot Project (2010) Delmarva Power—An Exelon Company https://www.delmarva.com/News/Pages/NewsReleases/2010-03-24.aspx
Didham RK, Basset Y, Collins CM, Leather SR, Littlewood NA, Menz MHM, Müller J, Packer L, Saunders ME, Schönrogge K, Stewart AJA, Yanoviak SP, Hassall C (2020) Interpreting insect declines: seven challenges and a way forward. Insect Conserv Divers 13(2):103–114
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B (2014) Defaunation in the Anthropocene. Science 345(6195):401–406
Dively GP, Venugopal PD, Bean D, Whalen J, Holmstrom K, Kuhar TP, Doughty HB, Patton T, Cissel W, Hutchison WD (2018) Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc Natl Acad Sci 115(13):3320–3325
Donners M, van Grunsven RHA, Groenendijk D, van Langevelde F, Bikker JW, Longcore T, Veenendaal E (2018) Colors of attraction: modeling insect flight to light behavior. J Experimental Zool A 329(8–9):434–440
Eisenbeis G (2006) Artificial night lighting and insects: attraction of insects to streetlamps in a rural setting in Germany. In: Rich C, Longcore T (eds) Ecological Consequences of Artificial Night Lighting. Island, pp 281–304
Elvidge CD, Baugh KE, Kihn EA, Kroehl HW, Davis ER (1997) Mapping city lights with nighttime data from the DMSP operational Linescan System. Photogram Eng Remote Sens 63(6):727–734
Elvidge CD, Baugh K, Zhizhin M, Hsu FC, Ghosh T (2017) VIIRS night-time lights. Int J Remote Sens 38(21):5860–5879
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat 139:S125–S153
Fabian ST, Sondhi Y, Allen P, Theobald J, Lin H-T (2024) Why flying insects gather at artificial light. Nat Commun 15:689
Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R (2016) The new world atlas of artificial night sky brightness. Sci Adv 2:e1600377
Farias JR, Costa EC, Guedes JVC, Arbage AP, Neto AP, Bigolin M, Pinto FF (2013) Managing the sugarcane borer, Diatraea Saccharalis, and corn earworm, Helicoverpa zea, using Bt corn and insecticide treatments. J Insect Sci 13(1):109
Fiorentin P, Binotto R, Cavazzani S, Bertolo A, Ortolani S, Saviane I (2022) Long-time trends in night sky brightness and ageing of SQM radiometers. Remote Sens, 14(22)
Fitt GP (1989) The ecology of Heliothis species in relation to agroecosystems. Ann Rev Entomol 34(1):17–53
Forister ML, Halsch CA, Nice CC, Fordyce JA, Dilts TE, Oliver JC, Prudic KL, Shapiro AM, Wilson JK, Glassberg J (2021) Fewer butterflies seen by community scientists across the warming and drying landscapes of the American West. Science 371(6533):1042–1045
Fox J, Weisberg S (2019) An R companion to Applied Regression. Sage, Thousand Oaks CA
Fox R, Dennis EB, Harrower CA, Blumgart D, Bell JR, Shortall CR, Bell JR, Cook P, Davis AM, Evans-Hill LJ, Haynes F, Hill D, Isaac NJB, Parsons MS, Pocock MJO, Prescott T, Randle Z, Shortall CR, Tordoff GM, Tuson D, Bourn NAD (2021) The state of Britain’s larger moths 2021. Butterfly Conservation, Rothamsted Research and UK Centre for Ecology & Hydrology
Frank KD (2006) Effects of Artificial Night Lighting on moths. In: Rich C, Longcore T (eds) Ecological consequences of Artificial Night Lighting. Island, pp 305–344
Garamszegi LZ, Eens M, Török J (2009) Behavioural syndromes and trappability in free-living collared flycatchers, Ficedula albicollis. Anim Behav 77(4):803–812
Garris HW, Snyder JA (2010) Sex-specific attraction of moth species to ultraviolet light traps. Southeast Nat 9(3):427–434
Gassmann AJ, Reisig DD (2023) Management of insect pests with Bt crops in the United States. Ann Rev Entomol 68:31–49
Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R (2017) Google Earth Engine: planetary-scale geospatial analysis for everyone. Remote Sens Environ 202:18–27
Grenis K, Nufio C, Wimp GM, Murphy SM (2023) Does artificial light at night alter moth community composition? Philosophical Trans Royal Soc B 378(1892):20220365
Guetté A, Godet L, Juigner M, Robin M (2018) Worldwide increase in Artificial Light at Night around protected areas and within biodiversity hotspots. Biol Conserv 223:97–103
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, de Kroon H (2017) More than 75% decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12(10):e0185809
Hartstack AW, Witz JA, Buck DR (1979) Moth traps for the tobacco budworm. J Econ Entomol 72(4):519–522
Harvey JA, Heinen R, Armbrecht I, Basset Y, Baxter-Gilbert JH, Bezemer TM, Böhm M, Bommarco R, Borges PAV, Cardoso P, Clausnitzer V, Cornelisse T, Crone EE, Dicke M, Dijkstra K-DB, Dyer L, Ellers J, Fartmann T, Forister ML, ..., de Kroon H (2020) International scientists formulate a roadmap for insect conservation and recovery. Nat Ecol Evol 4(2):174–176
Haynes KJ, Robertson BA (2021) A transdisciplinary research agenda for understanding insect responses to ecological light pollution informed by evolutionary trap theory. Curr Opin Insect Sci 45:91–96
Hordley LA, Fox R, Suggitt AJ, Bourn NAD (2023) Precipitation buffers temperature-driven local extinctions of moths at warm range margins. Ecol Lett 26(5):805–815
Hsiao HS (1973) Flight paths of night-flying moths to light. J Insect Physiol 19(10):1971–1976
Hung L-W, Anderson SJ, Pipkin A, Fristrup K (2021) Changes in night sky brightness after a countywide LED retrofit. J Environ Manage 292:112776
Janzen DH, Hallwachs W (2019) Perspective: where might be many tropical insects? Biol Conserv 233:102–108
Jonason D, Franzén M, Ranius T (2014) Surveying moths using light traps: effects of weather and time of year. PLoS ONE, 9(3), e92453
Kawahara AY, Reeves LE, Barber JR, Black SH (2021) Opinion: eight simple actions that individuals can take to save insects from global declines. Proc Natl Acad Sci 118(2):e2002547117
Kyba CCM (2016) Defense Meteorological Satellite Program data should no longer be used for epidemiological studies. Chronobiol Int 33:943–945
Kyba CCM, Tong KP, Bennie J, Birriel I, Birriel JJ, Cool A, Danielsen A, Davies TW, Outer PND, Edwards W, Ehlert R, Falchi F, Fischer J, Giacomelli A, Giubbilini F, Haaima M, Hesse C, Heygster G, Hölker F, Gaston KJ (2015) Worldwide variations in artificial skyglow. Sci Rep 5(1):8409
Kyba CCM, Aubé M, Bará S, Bertolo A, Bouroussis CA, Cavazzani S, Espey BR, Falchi F, Gyuk G, Jechow A, Kocifaj M, Kolláth Z, Lamphar H, Levin N, Liu S, Miller SD, Ortolani S, Pun CSJ, Ribas SJ, ..., Zhang J (2022) Multiple angle observations would benefit visible band remote sensing using night lights. J Phys Res, 127(12), e2021JD036382.
Kyba CCM, Altıntaş YÖ, Walker CE, Newhouse M (2023) Citizen scientists report global rapid reductions in the visibility of stars from 2011 to 2022. Science 379(6629):265–268
Lawton D, Huseth AS, Kennedy, Kennedy GG, Morey AC, Hutchison WD, Reisig DD, Dorman SJ, Dillard D, Venette RC, Groves RL, Adamczyk JJ, Barbosa Dos Santos I, Baute T, Brown S, Burkness E, Dean A, Dively GP, Doughty HB, ..., Zuefle M (2022) Pest population dynamics are related to a continental overwintering gradient. Proceedings of the National Academy of Sciences, 119(37), e2203230119
Leather S (2015) Entomological classics – The light trap. Don’t Forget the Roundabouts. https://simonleather.wordpress.com/2015/08/06/entomological-classics-the-light-trap/
Leather S (2018) Ecological armageddon – more evidence for the drastic decline in insect numbers. Ann Appl Biol 172(1):1–3
Lenth RV (2023) emmeans: Estimated Marginal Means, aka Least-Squares Means. https://CRAN.R-project.org/package=emmeans
Li X, Zhou Y, Zhao M, Zhao X (2020) A harmonized global nighttime light dataset 1992–2018. Sci Data, 7(1)
Lister BC, Garcia A (2018) Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc Natl Acad Sci 115(44):e10397–e10406
Loboda S, Savage J, Buddle CM, Schmidt NM, Høye TT (2018) Declining diversity and abundance of high Arctic fly assemblages over two decades of rapid climate warming. Ecography 41(2):265–277
Longcore T, Rich C (2004) Ecological light pollution. Front Ecol Environ 2(4):191–198
Martín-Vega D, Baz A (2013) Sex-biased captures of sarcosaprophagous Diptera in carrion-baited traps. J Insect Sci 13(1):14
McDermott EG, Mullens BA (2018) The dark side of light traps. J Med Entomol 55(2):251–261
Merckx T, Slade EM (2014) Macro-moth families differ in their attraction to light: implications for light-trap monitoring programmes. Insect Conserv Divers 7(5):453–461
Niemelä PT, Lattenkamp EZ, Dingemanse NJ (2015) Personality-related survival and sampling bias in wild cricket nymphs. Behav Ecol 26(3):936–946
Nowinszky L, Puskás J (2012) Light-trap catch of the harmful moths depending of moonlight in North Carolina and Nebraska states of USA. Int Sch Res Notices 2012:e238591
Owens ACS, Cochard P, Durrant J, Farnworth B, Perkin EK, Seymoure B (2020) Light pollution is a driver of insect declines. Biol Conserv 241:108259
Owens ACS, Van den Broeck M, De Cock R, Lewis SM (2022) Behavioral responses of bioluminescent fireflies to artificial light at night. Front Ecol Evol, 10
Reay-Jones FPF, Bilbo TR, Reisig DD (2020) Decline in sublethal effects of Bt corn on corn earworm (Lepidoptera: Noctuidae) linked to increasing levels of resistance. J Econ Entomol 113(5):2241–2249
Rhoades P, Griswold T, Waits L, Bosque-Pérez NA, Kennedy CM, Eigenbrode SD (2017) Sampling technique affects detection of habitat factors influencing wild bee communities. J Insect Conserv 21(4):703–714
Seymour M, Perera OP, Fescemyer HW, Jackson RE, Fleischer SJ, Abel CA (2016) Peripheral genetic structure of Helicoverpa zea indicates asymmetrical panmixia. Ecol Evol 6(10):3198–3207
Simons AL, Yin X, Longcore T (2020) High correlation but high scale-dependent variance between satellite measured night lights and terrestrial exposure. Environmental Research Communications, 2
Slingerland MV (1902) Trap-lanterns or Moth catchers. Cornell University
Somers-Yeates R, Hodgson D, McGregor PK, Spalding A, ffrench-Constant RH (2013) Shedding light on moths: shorter wavelengths attract noctuids more than geometrids. Biol Lett 9(4):20130376
Taylor RJ (1986) Time series analysis of numbers of Lepidoptera caught at light traps in East Africa, and the effect of moonlight on trap efficiency. Bull Entomol Res 76(4):593–606
Thomas CD, Jones TH, Hartley SE (2019) Insectageddon: a call for more robust data and rigorous analyses. Glob Change Biol 25(6):1891–1892
Van de Schoot E, Merckx T, Ebert D, Wesselingh RA, Altermatt F, Van Dyck H (2024) Evolutionary change in flight-to-light response in urban moths comes with changes in wing morphology. Biol Lett 20:20230486
van der Kooi CJ, Stavenga DG, Arikawa K, Belušič G, Kelber A (2021) Evolution of insect color vision: from spectral sensitivity to visual ecology. Ann Rev Entomol 66:435–461
van Langevelde F, Ettema JA, Donners M, WallisDeVries MF, Groenendijk D (2011) Effect of spectral composition of artificial light on the attraction of moths. Biol Conserv 144(9):2274–2281
Verheijen FJ (1958) The mechanisms of the trapping effect of artificial light sources upon animals. Archives Néerlandaises De Zool 13:1–107
Wagner DL (2019) Global insect decline: Comments on Sánchez-Bayo and Wyckhuys (2019). Biological Conservation, 233, 332–333
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021a) Insect decline in the Anthropocene: Death by a thousand cuts. Proceedings of the National Academy of Sciences, 118(2)
Wagner DL, Fox R, Salcido DM, Dyer LA (2021b) A window to the world of global insect declines: Moth biodiversity trends are complex and heterogeneous. Proceedings of the National Academy of Sciences, 118(2)
Westbrook JK, López JD (2010) Long-distance migration in Helicoverpa zea: what we know and need to know. Southwest Entomol 35(3):355–360
White PJT (2018) An aerial approach to investigating the relationship between macromoths and artificial nighttime lights across an urban landscape. J Agricultural Urban Entomol 34(1):1–14
White PJT, Glover K, Stewart J, Rice A (2016) The technical and performance characteristics of a low-cost, simply constructed, black light moth trap. J Insect Sci 16(1):25
Williams CB (1936) IX—The influence of moonlight on the activity of certain nocturnal insects, particularly of the family Noctuidae, as indicated by a light trap. Philosophical Trans Royal Soc B 226(537):357–389
Wölfling M, Becker MC, Uhl B, Traub A, Fiedler K (2016) How differences in the settling behaviour of moths (Lepidoptera) may contribute to sampling bias when using automated light traps. Eur J Entomol 113(1):502–506
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J Royal Stat Soc B 73(1):3–36
Yazdanian M, Kankaanpää T, Leinonen ItämiesJ, Merckx R, Pöyry T, Sihvonen J, Suuronen P, Välimäki A, P., Kivelä SM (2023) Ecological and life-history traits predict temporal trends in biomass of boreal moths. Insect Conserv Divers 16:600–615
Yela JL, Holyoak M (1997) Effects of moonlight and meteorological factors on light and bait trap catches of noctuid moths (Lepidoptera: Noctuidae). Environ Entomol 26(6):1283–1290
Zissis G, Bertoldi P, Serrenho T (2021) Update on the status of LED-lighting world market since 2018. Publications Office of the European Union
Acknowledgements
We are grateful to the many farmers who collaborated with the University of Delaware, Rutgers University, and University of Minnesota corn earworm monitoring networks. We would also like to thank Jacob Dayton and Wendy A. Valencia-Montoya as well as Vaidehi Chotai, Ross Vieira, and the rest of the Owens-Gozzi joint lab meeting for their valuable feedback on this study.
Author information
Authors and Affiliations
Contributions
IB: Conceptualization, Software, Formal analysis, Visualization, Data curation, Writing – Original draft. EB, MSC, KH, WH, JI, DO: Investigation, Resources, Writing – Review and editing, Funding acquisition. CBE: Formal analysis, Data curation, Validation, Writing – Review and editing. ACSO: Conceptualization, Methodology, Validation, Visualization, Supervision, Writing – Review and editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1:
Figure S1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Battles, I., Burkness, E., Crossley, M.S. et al. Moths are less attracted to light traps than they used to be. J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00588-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00588-x