Abstract
Heterogeneity at local and landscape scales can promote insect diversity and moderate insect declines that stem from global change. Determining how species respond to different landscape components provides insight into the role of heterogeneity in landscapes undergoing change. We examine how indigenous forest-grassland edges are used by butterflies. We assessed butterfly diversity and behaviour at forest edges and adjoining grassland, and tested whether these patterns are influenced by differing weather conditions between seasons. Forest edges supported a species rich butterfly assemblage. Forest specialists were more diverse at forest edges than in grassland, whereas grassland specialists and habitat generalists were as diverse at forest edges as in grassland. All butterfly groups showed more inter- and intra-specific interactions and more patrolling behaviour at forest edges, but more feeding and transient behaviour in grassland. Occurrence and behavioural patterns were not mediated by season, suggesting that the influence of forests does not change with varying environmental conditions. Nonetheless, certain species preferentially utilized forest edges in the hot and windy season, indicating that shelter provided by forest edges influences butterfly habitat use. We found no evidence that complementary nectar sources influence butterfly distribution patterns.
Implications for insect conservation: The diverse butterfly assemblages and range of behaviours supported by indigenous forest edges indicate that forest patches are an important habitat component for butterflies. Conserving forest patches in these coastal grasslands may help buffer butterfly populations against global change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solutions are needed to mitigate global insect declines due to a range of anthropogenic stressors such as habitat loss, climate change and land-use intensification (Cardoso et al. 2020; Harvey et al. 2022). The maintenance of spatial heterogeneity at local and landscape scales is a primary strategy towards moderating insect losses (Samways et al. 2020; Seibold et al. 2019). Relationships between heterogeneity and the diversity of ecological communities can be complex due to trade-offs between suitable habitat area and heterogeneity (Allouche et al. 2012; Heidrich et al. 2020). However, in many cases, heterogeneity increases insect diversity in various ways.
At the community level, heterogeneity provides diverse ecological niches which allow species with different habitat requirements to coexist (Fahriget al. 2011). For example, different vegetation types, heterogeneous vegetation structure, environmental gradients, and small natural features can increase species richness (Hunter et al. 2017; Löffler and Fartmann 2017; Pryke et al. 2013). Individual species also benefit from heterogeneity, as many species need a wide range of resources to fulfil their ecological and behavioural needs. This includes consumable resources such as larval host plants and adult food, as well as structural elements such as bare ground or tall vegetation that are needed for roosting, overwintering, egg-laying, and mate location (Dennis et al. 2003; Schultz et al. 2012). These varied resources often do not co-occur in the same biotope, and a mix of biotopes within a species’ dispersal range can be a prerequisite for population persistence (Kalarus et al. 2013; Slamova et al. 2013).
In addition, individual vegetation types undergo gradual changes in the phenology of their food resources throughout the season, and seldom provide a continuous food supply over time. However, different landscape elements can provide phenological complementarity in food resources that allows species to persist throughout the season by tracking seasonal patterns in resource availability (Bertrand et al. 2019; Mallinger et al. 2016; Mandelik et al. 2012). Furthermore, structurally diverse landscapes provide shelter during adverse weather conditions and refuge from human disturbances (Dennis and Sparks 2005, 2006; Marini et al. 2009). By using refuges, species can remain active for longer periods and persist in areas that would otherwise be unsuitable under fluctuating environmental conditions (Dennis and Sparks 2005, 2006; Fartmann 2006). In this way, these refuges boost the resilience of arthropod populations to global change (Fartmann et al. 2022; Selwood and Zimmer 2020).
Indigenous forest patches and forest edges can increase the structural and compositional complexity of open ecosystems such as grasslands (Akeboshi et al. 2015). In many regions, grassland insect assemblages benefit from the presence of indigenous forest (Bergman et al. 2018; Marini et al. 2009; Öckinger et al. 2012), and in some cases, forests close to grassland can buffer the negative effects of habitat loss and fragmentation on grassland insects (Öckinger et al. 2012). Supplementary food resources, shelter, and a diversity of microclimates at forest edges, are often proposed as reasons for these positive effects (Habel et al. 2022; Liivamägi et al. 2014; Toivonen et al. 2017).
In South Africa, indigenous forest patches embedded in grassland ecosystems have high conservation value, but are limited in extent, with the forest biome covering < 1% of land area in South Africa (Mucina and Rutherford 2006). Forest patches support specialist forest arthropods, and assemblages that complement those in grassland (Eckert et al. 2022; Gaigher et al. 2019; Yekwayo et al. 2017). Forests also influence insect assemblages in nearby grassland (Pryke and Samways 2012). This latter effect is especially evident for butterflies, which are responsive to amount and configuration of woody vegetation at the landscape scale (van Schalkwyk et al. 2021). In these systems, certain butterflies in grassland benefit from forest cover in the landscape, even in highly transformed landscapes, suggesting that they utilize the forests and/or their edges (Gaigher et al. 2021). However, the different ways in which these forests are used by grassland species have not been assessed in detail. As the influence of forests on grassland insects has important implications for insect species distribution and persistence, a more detailed understanding of the mechanisms behind these influences will enable us to predict the role that forests may play in changing landscapes.
Here, we evaluate the extent to which indigenous forest edges are utilized by grassland butterflies and investigate two potential benefits provided by forest edges: shelter from adverse weather conditions and complementary adult nectar sources, both factors which can strongly influence butterfly distribution and behaviour (Dennis and Sparks 2005, 2006; Evans et al. 2020; Fischer and Fiedler 2001). First, we assess patterns of butterfly diversity at forest edges and adjacent grassland. We expect species with different habitat preferences to respond in different ways to forest edges and open grassland, and therefore we assess patterns for forest specialists, grassland specialists, and habitat generalist butterflies separately. High butterfly diversity at forest edges, especially of grassland specialists, would indicate the importance of forest edges for supporting species other than the expected forest-associated species. In addition to promoting biodiversity, certain landscape elements can be preferentially used for certain behaviours and can thus be important for population persistence (Crous et al. 2014; Evans et al. 2020; Pryke et al. 2012). We therefore also assess whether butterfly behaviour differs between forest edges and adjacent grassland. Furthermore, we compare single-species abundance patterns of the dominant butterfly species at forest edges and in grassland.
We also test for the effect of season as a proxy for coarse-scale climatic conditions, as the average conditions between the two sampling seasons of this study differ greatly, with summer having significantly higher wind speeds, air temperature, insolation, and significantly lower relative humidity than is the case in autumn. A mediating effect of season on the occurrence and behaviour patterns would indicate that prevailing weather conditions influence the relative importance of the forest edge as a component of butterfly habitat. This would help us to determine whether forest edges provide shelter from adverse conditions, which we expected in our high-wind and high-temperature study area. It would also help explain any species-level shifts in habitat use between seasons. Finally, we assess whether flowering plants that butterflies visit differ between forest edges and grassland, and between seasons, to determine whether the two biotopes support complementary adult food sources which may influence butterfly habitat use.
Methods
Study design
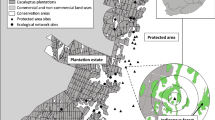
The study area is located on the east coast of South Africa in the Maputaland-Pondoland-Albany biodiversity hotspot (Myers et al. 2000). The landscape is a flat, coastal plain which is dominated by two vegetation types that exist as an intricate mosaic: Maputaland Coastal Belt and Maputaland Wooded Grassland, which, in the study area, consists largely of dry and hygrophilous grassland with scattered shrubs and small trees. Grassland sites had an average of 8.52% ± 1.53 S.E. shrub cover and an average of 3.98% ± 0.89 S.E. tree cover. Small patches of Northern Coastal Forest and Swamp Forest are embedded in the grassland matrix. These are medium-height, subtropical, coastal forests with high plant species richness (Mucina et al. 2006). The forest-grassland edges are soft, consisting of transition vegetation, with the ecotones ranging from 2 to 20 m in width. Forest edge sites had an average of 20.78% ± 3.31 S.E. shrub cover and an average of 36.56% ± 3.60 S.E. tree cover. Sixteen sites were selected where forest patches > 2.5 ha were next to open grassland (Fig. 1). Both indigenous forest types (Northern Coastal Forest: 11 sites, Swamp Forest: 5 sites) and both grassland types (Maputaland Coastal Belt: 9 sites, Maputaland Wooded Grassland: 7 sites) were included (Scott-Shaw and Escott, 2011). Eight of the sites were in large-scale conservation corridors in a forestry plantation estate owned by SiyaQhubeka Forestry and managed by Mondi Group. The production areas of the plantations are planted monocultures of Eucalyptus with limited understory vegetation. Another eight sites were in the adjacent protected area, iSimangaliso Wetland Park, east of the plantations. Distances between sites ranged from 600 m to 11 km. The grassland in the protected area and the conservation corridors undergo similar fire regimes, with prescribed burns every 2–5 years to mimic natural fire disturbances (SANBI 2014). Grazing between the areas is also similar, as the border between them is unfenced, which allows wild megaherbivores to move freely and graze the vegetation in both areas. Coarse vegetation structure and composition during the study period was similar between grasslands of the two areas (Gaigher et al. 2021).
Sampling methods
Butterflies were sampled twice at each site, once during 19–25 January 2017 (summer) and once during 27 April-5 May 2017 (autumn). Both seasons are periods of high activity for subtropical butterflies in the region (Woodhall 2005). To characterise the weather conditions in the two study periods, hourly weather data on solar radiation and rainfall were obtained from a weather station close to the study area (28˚21’08.56”S, 32˚14’46.42”E) and hourly data on wind speed, air temperature, and relative humidity were obtained from the ERA5 global weather dataset, and were derived from a point close to the study area (28˚15’00.01”S, 32˚14’59.97”E) (Hersbach et al. 2020). Linear models were performed in lme4 (Bates et al. 2014) to test whether these measures differed significantly between the two study periods. Wind speed, air temperature and solar radiation were significantly higher in summer, whereas relative humidity was higher in autumn, and rainfall did not differ between seasons (Supplementary material, Table S1).
We sampled butterflies between 08h00 and 15h00 and avoided overcast, rainy, and very windy conditions (wind speeds of > 10 m/s), although it was not possible to avoid windy conditions altogether. At each site, butterflies were recorded along two transects. One transect was along the forest edge in the transition zone which consisted of a mix of grassland and forest vegetation, and one transect was in the adjacent grassland between 50 and 150 m from the forest edge. Sampling in the forest interiors was not possible due to safety restrictions in these areas with free-roaming wild megaherbivores. Each transect consisted of two observers simultaneously walking along a slow, meandering route, recording all butterflies in a 5 m radius along the transect. Observers walked in opposite directions and stayed in contact to avoid recording the same individual twice. In total, each transect lasted for 30 min (Kadlec et al. 2012), adding up to one hour of observations per transect per season, and was approximately 300 m long in total. We surveyed the ground vegetation layer, and if shrubs and trees were present at a site, we also surveyed the shrubs, and lower tree canopies. The behaviour of each butterfly when it was first sighted was recorded and classified as transient (high, fast flight), patrolling (low, slow, searching flight), settling behaviour (resting and basking), feeding, and inter- and intraspecific interactions (territorial and reproductive behaviour) (Dover 1989). For all feeding events, we identified the flowering plants visited using Pooley (1993; 1998). Butterflies that could not be identified in flight were captured for identification according to Pennington et al. (1978) and Woodhall (2005). Species were classified as forest specialists, grassland specialists, or habitat generalists, based on historical data on their occurrence in different vegetation types (Supplementary material, Table S2) (Mecenero et al. 2013).
Statistical analysis
All records of the Boisduval tree nymph, Sevenia boisduvali boisduvali, were removed from the dataset, as this species shows periodic swarming behaviour in the study region (Mecenero et al. 2013) and there was great variation in its abundance among sites. An outlier site in the plantation estate was excluded from analysis due to elevated butterfly numbers caused by a mass-flowering event in summer (Zuur et al. 2010). All analyses were done in R version 4.2.0 (R Core Team 2022). To assess sampling adequacy, individual-based rarefaction and extrapolation sampling curves were made using the iNEXT package (Hsieh et al. 2016).
We performed generalized linear mixed models using the glmmTMB package (Brooks et al. 2017) to test for the effect of location in the landscape (forest edge or grassland), season (summer or autumn), and their interaction on species richness and abundance of the overall assemblage, and that of forest specialists, grassland specialists, and habitat generalists. Poisson distribution was used for species richness models, and negative binomial distribution for abundance models (Bolker et al. 2009). In all models, location, season, and their interaction were included as fixed factors. We also included area (protected area or plantation estate) as a fixed factor to assess possible effects of the larger landscape setting and transect as a random factor to account for repeated measurements at the same location (Bolker et al. 2009). Model selection and averaging were used to identify the most influential set of variables for each response variable (Burnham and Anderson 2001). Models consisting of all possible combinations of variables, including the null model, were ranked based on AICc in the MuMIn package (Barton 2022). All models within 2 AICc of the top model were averaged and estimates from conditional model averaging are reported. Effects were considered significant if the 95% confidence intervals of the estimate did not include zero (Burnham and Anderson 2001). Significant interactions were further assessed by testing for the effect of location or season on the responses separated by season or location, respectively.
To assess differences in behaviours between locations and seasons, we converted the number of occurrences per behavioural category to a proportion of the total number of behavioural occurrences per transect and used this as response variable in models. This was done because the absolute number of behavioural occurrences is dependent on the number of butterfly individuals recorded. The same modelling procedure was used as above, except that we used linear mixed models because the proportions were continuous, non-binomial data and best fitted a Gaussian distribution (Bolker et al. 2009; Warton and Hui 2011). This procedure was done for the overall butterfly assemblage, and for forest specialists, grassland specialists, and generalists separately.
The same modelling procedure as above was used to assess abundance patterns of dominant species (here defined as species that were observed more than ten times per season) between locations and seasons. We used negative binomial distribution for these abundance models (Bolker et al. 2009). For all models, we checked for overdispersion, and assessed the model fit of alternative models by visualizing the model residuals using the DHARMa package (Hartig 2020). We confirmed there was no spatial autocorrelation in the model residuals by assessing correlograms using the ncf package (Bjornstad and Cai 2020).
Changes in butterfly assemblage composition due to differences in species phenology between seasons may have influenced the abovementioned occurrence and behavioural patterns between forest edges and grassland. To evaluate this possibility, we assessed differences in the butterfly assemblages occurring in summer and autumn using the manyglm function in mvabund (Wang et al. 2012). This model-based procedure fits generalized linear models separately to the abundance of each species and was used due to its greater power properties compared to more commonly used distance-based approaches (Wang et al. 2012; Warton et al. 2012). The multivariate model included season as fixed effect and was fitted using a negative binomial distribution, assuming quadratic mean-variance. Test statistics were calculated based on the ‘PIT-trap’ resampling method with 999 permutations (Wang et al. 2012).
We used the same multivariate procedure to test whether the flowering plant assemblages visited by butterflies differed between forest edges and grassland, and between seasons. The model included location, season, their interaction, and area as fixed factors. The response matrix consisted of the number of butterfly visitations per visited flowering plant species per site. We did not include all flowering plant species present at the sites, but only those that were visited, because we could confirm that they were utilized by butterflies.
Results
We sampled a total of 2 572 butterflies in 65 species (Supplementary material, Table S2). From this, 846 butterflies in 48 species were sampled in summer and 1726 butterflies in 52 species were sampled in autumn. In total, 20 species were forest specialists, 14 were grassland specialists, and 31 were habitat generalists. The rarefaction curve for forest edge sampling approaches an asymptote, whereas the curve for grassland sites still increases, indicating that additional species would be recorded with further sampling (Supplementary material, Fig. S1).
For the overall assemblage, butterfly species richness and abundance were significantly higher at the forest edges than in grassland and were significantly higher in autumn than in summer (Table 1; Fig. 2A-B). Species richness of forest specialists was higher at the forest edges than in grassland (Table 1; Fig. 2C). The abundance of forest specialists showed an interaction between location and season, with lower abundance in grassland than at forest edges in summer (estimate: -1.66, S.E.: 0.35, 95% C.I.: -2.39, -0.94), but not in autumn (null model performed best) (Table 1; Fig. 2D). Grassland specialists and habitat generalists were equally species rich and abundant at forest edges and in grassland, with both groups more species rich and abundant in autumn than in summer (Table 1; Fig. 2E-H). Area did not have a significant effect on any of the butterfly responses (Table 1).
Butterfly species richness and abundance at forest edges and in grassland in summer and autumn for A-B) the overall assemblage, C-D) forest specialists, E-F) grassland specialists and G-H) habitat generalists. Horizontal brackets indicate significant main effects of location, vertical brackets indicate significant main effects of season. For the significant interaction in D, means with letters in common are not significantly different. Letters in roman relate to the comparison between locations in autumn, and letters in italics relate to the comparison between locations in summer. The boxplots represent the median (central horizontal line), inter-quantile range (boxes), minimum and maximum values (whiskers) and outliers (points)
For the overall butterfly assemblage, the number of settling behavioural events was similar between forest edges and grassland but was significantly higher in summer than in autumn (Table 2; Fig. 3). There were more feeding behavioural events in the open grassland than at forest edges (Table 2; Fig. 3). There were more intra- and interspecific interactions at the forest edges than in grassland, and more in summer than in autumn (Table 2; Fig. 3). Patrolling was more frequent at the forest edges than in grassland and was more frequent in autumn than in summer (Table 2; Fig. 3). There were more transient behavioural events in the grassland than at forest edges, and more in summer than in autumn (Table 2; Fig. 3). There were no significant interactions between location and season, and no effect of area on any of the behavioural categories. Trends in behaviour of the three butterfly groupings in the different locations and seasons were similar to the overall assemblage, except that the grassland specialists showed fewer overall transient behaviours, but more settling and feeding behaviours than the other butterfly groups, and forest specialists showed fewer overall settling and feeding behavioural events than the other butterfly groups (Supplementary material, Figure S2).
Of the dominant forest specialist species, the novice, Amauris ochlea ochlea, and the mocker swallowtail, Papilio dardanus cenea, were significantly more abundant at forest edges than in grassland across both seasons (Table 3; Supplementary material, Table S3). The layman, Amauris albimaculata albimaculata, was significantly more abundant at forest edges than in grassland in summer (estimate: -3.32, S.E.: 1.61, 95% C.I.: -6.48, -0.16), but did not differ between locations in autumn (estimate: -0.43, S.E.: 0.45, 95% C.I.: -1.16, 0.30) (Table 3; Supplementary material, Table S3). The small orange Acraea, Hyalites eponina, was not influenced by location (Table 3; Supplementary material, Table S3). Of the dominant grassland specialists, the spotted joker, Byblia ilithyia, was significantly more abundant at forest edges than in grassland in both seasons (Table 3; Supplementary material, Table S3). Neither the common grass yellow, Eurema hecabe solifera, nor the broad-bordered grass yellow, Eurema brigitta brigitta, were influenced by location (Table 3; Supplementary material, Table S3). The abundance of the blue pansy, Junonia oenone oenone, was not influenced by any of the predictors (Table 3; Supplementary material, Table S3). Of the dominant habitat generalists, the citrus swallowtail, Papilio demodicus demodicus, was significantly more abundant at forest edges than in grassland in summer (estimate: -1.40, S.E.: 0.40, 95% C.I.: -2.27, -0.54), but its abundance did not differ between locations in autumn (estimate: -0.36, S.E.: 0.23, 95% C.I.: -0.85, 0.12) (Table 3; Supplementary material, Table S3). The window Acraea, Acraea oncaea, was influenced only by area, being significantly more abundant in grassland sites on the plantation estate (Table 3; Supplementary material, Table S3). The abundance of the four remaining habitat generalists, Barker’s smoky blue, Euchrysops barkeri, African monarch, Danaus chrysippus aegyptius, African migrant, Catopsilia florella, and African common white, Belenois creona severina, were not influenced by any of the predictors (Table 3; Supplementary material, Table S3).
Although many of the same butterfly species occurred in both seasons (Table S2), there was a significant difference in butterfly assemblage composition between summer and autumn (Deviance = 375.1, P = 0.001).
In total, 201 flower visitations to 48 flowering plant species were recorded. In summer, there were 56 visitations to 21 flowering plant species, and in autumn there were 145 visitations to 36 flowering plant species. The flowering plants visited were a mix of herbaceous and woody plants (Supplementary material, Table S3). The plant assemblage visited did not differ between forest edges and grassland (Wald χ2 = 2.52, P = 0.47), between seasons (Wald χ2 = 2.73, P = 0.68), or between the protected area and plantation estate (Wald χ2 = 3.30, P = 0.49), and there was no interaction between location and season (Wald χ2 = 0.72, P = 0.41).
Discussion
We found that indigenous forest edges are important habitat components for butterflies, supporting rich butterfly assemblages consisting of a mix of forest specialists, habitat generalists, and grassland specialists. Certain behaviours indicative of habitat use, such as territory defence and courtship behaviour, were more prevalent at forest edges than in grassland. Furthermore, certain species preferentially used forest edges, especially during hotter and windier summer conditions. Forest edges therefore appear to increase habitat complexity and provide refuge for butterflies in the study region.
Importance of forest edges for maintaining high butterfly diversity and supporting certain behaviours
Edges between forests and open habitats often support diverse arthropod assemblages (Schmitt et al. 2020; Yekwayo et al. 2016), and this was also observed here. The high diversity at forest edges was driven largely by high numbers of forest specialists. Yet, grassland specialists and habitat generalists were equally species rich and abundant at the forest edges compared to grassland. Although certain grassland butterflies favour expansive unwooded grasslands (Gaigher et al. 2021; Schmitt and Seitz 2000), our results show that a significant component of the overall local butterfly assemblage utilizes forest edges.
Edges between forests and open areas often benefit arthropods, as they provide easy access to resources from both adjacent biotopes (Ries et al. 2004) and have gradients of solar radiation, temperature, and moisture that provide varied microhabitats supporting species with different microclimatic needs (Liivamägi et al. 2014; Schultz et al. 2012). In addition, these ecotones can provide rich floral resources (Bergman et al. 2018), dense larval host plants (Habel et al. 2022), and protection from adverse weather such as strong winds (Toivonen et al. 2017) and extreme temperatures (van Halder et al. 2011). In this high-wind and hot environment, we expected shelter from harsh weather to strongly influence butterfly distribution and behaviour. Such a sheltering effect would have been evident if butterfly species richness and abundance patterns in the different landscape locations were mediated by the effect of season, or if settling behaviours were more frequent at forest edges than in grassland. Such patterns have been demonstrated for butterflies and grasshoppers that shift in their microsite selection according to shifting weather conditions (Dennis and Sparks 2006; Dover et al. 1997; Matenaar et al. 2014).
We did not observe these effects, but instead saw similar butterfly patterns between summer and autumn for all three butterfly groups. A possible reason for this may be that our use of the average seasonal conditions was too coarse to detect effects of weather conditions which can vary over shorter time scales. Furthermore, butterfly assemblage composition differed between seasons, and varying climatological preferences of different species occurring in the different seasons may have partly masked the occurrence and behavioural patterns at the assemblage level. Nonetheless, the single species patterns provided an indication that forest edges provide refuge for certain species. For example, A. albimaculata albimaculata and P. demodicus demodicus, preferred forest edges over grassland during summer, whereas they utilized forest edges and grassland equally during autumn. These are both mobile strong-flying butterflies which may benefit from the ability to actively seek out sheltered areas during harsher periods to avoid the effects of the wind, such as convective heat loss and mechanical disturbance (Barton 2014; Matenaar et al. 2014), or to prevent overheating from high (> 30 °C) temperatures (van Halder et al. 2011). Open areas are then used more frequently during calmer conditions and moderate temperatures, which has also been shown for butterflies in other landscapes comprised of habitats that vary in structure (Dennis and Sparks 2006). These results suggest that forest edges provide important refuge opportunities, at least for certain butterfly species.
We also investigated whether forest edges provide abundant or complementary nectar sources to grassland, which benefits grassland butterflies in other regions (Bergman et al. 2018). However, we recorded more feeding behaviours in grasslands than at forest edges for grassland specialists and habitat generalists in both seasons. Few nectaring events were observed for forest specialists overall. A possible reason for the higher feeding activity in grassland may be higher floral abundance in grassland, which we did not measure directly in this study. Furthermore, we found no evidence of complementarity in the nectar sources between grassland and forest edges, as similar flower species were visited in both locations. This is contrary to other studies showing complementarity in pollinator food sources among different biotopes (Bertrand et al. 2019; Mallinger et al. 2016). It is therefore unlikely that adult nectar sources at forest edges are a reason for the edges being so highly utilized, at least in the lower vegetation layers. We were unable to survey flower visitations in the upper tree canopies, which may provide additional food sources.
In addition to flower visitations, the prevalence of other behaviours also varied between forest edges and grasslands. We observed proportionally more inter- and intra-species interactions at the forest edges than in grassland, which included territorial behaviours (aerial contests such as aggressive spiral flights and chases indicative of territory defence), courtship behaviours (slow, spiral flights between conspecifics), and mating. These patterns were consistent for all butterfly groups in both seasons. The high number of interactions at the forest edges may be because wooded habitats in predominantly open landscapes are clearly visible landmarks, which are often needed for visually cued butterfly behaviours like mate location and territory defence (Merckx and van Dyck 2005). Also, the heterogeneous conditions at forest edges increases an individual’s likelihood of locating a suitable microclimate. As microclimate is a crucial component of habitat quality for butterflies (Stuhldreher and Fartmann 2018), edges likely represent valuable territories for many species. By promoting these behaviours related to reproduction, forest edges may play an important role in preventing population decline and influencing the spatial distribution of subsequent butterfly generations (Dennis and Sparks 2006; Habel et al. 2022).
We also observed more patrolling (slow flight with high returning frequency) at the forest edges than in grassland, for all butterfly groups in both seasons. Although the motivation for patrolling varies between butterfly species, it generally relates to foraging, mate-location, and searching for shelter (Van Dyck and Baguette 2005) in habitats that are perceived as favourable (Evans et al. 2020; Schultz et al. 2012). Considering that proportions of total behaviours were analysed, the high frequency of nectar feeding in the grassland may partly explain why certain other behaviours were more frequent at forest edges. Nonetheless, the behavioural results show that the diversity of butterflies at the forest edges is not just due to passive mixing or accumulation at the edges (Ries et al. 2004), but that different butterfly groups are intentionally using forest edges as an important habitat element.
An additional factor which may have influenced butterfly occurrence at forest edges, is larval host plant distribution, which can strongly influence adult habitat selection (Habel et al. 2022; Krauss et al. 2005). This was not included in this assemblage-level study due to the huge diversity of host plants used by the various species (Woodhall 2005). Future work focused on single-species habitat use would be valuable to determine the role that host plant distribution plays in the observed butterfly occurrence patterns.
Importantly, although forest specialists were most abundant at forest edges, they also frequently utilized the grassland, mostly for transient behaviour (fast, directed flight associated with displacement) and patrolling, especially during the more moderate conditions in autumn. High-quality grassland remnants are important as habitat and as movement conduits for grassland butterflies in transformed landscapes (Pryke and Samways 2001), and our results suggest that they are also important for forest-associated butterflies. Conserving the integrity of the grassland surrounding forest patches is therefore crucial for maintaining forest butterfly diversity, which aligns with previous work on other taxonomic groups (Kotze and Samways 1999; Yekwayo et al. 2016).
Conservation implications
Indigenous forest edges are ecologically significant features for butterflies in these landscapes. They not only support a high diversity of forest-associated species, but also benefit butterflies in the broader grassland matrix. By providing shelter and varied microclimatic conditions, these forest edges increase opportunities for butterflies to satisfy their ecological and behavioural needs, which may become increasingly important under changing environmental conditions. Indeed, the maintenance of microhabitat diversity is identified as a primary management strategy towards buffering insect populations against global change (Harvey et al. 2022; Samways et al. 2020). Future research on the importance of forest patches and other landscape elements to long-term insect population persistence would help guide conservation planning in the study region. Furthermore, an understanding of the level of landscape heterogeneity that maximizes biodiversity would help fine-tune conservation action. Overall, our results indicate that the continued conservation of indigenous forest patches, along with the maintenance of a high-quality grassland matrix will be important for safeguarding overall butterfly diversity in these systems and in similar areas threatened by land-use change.
References
Akeboshi A, Takagi S, Murakami M, Hasegawa M, Miyashita T (2015) A forest–grassland boundary enhances patch quality for a grassland-dwelling butterfly as revealed by dispersal processes. J Insect Conserv 19:15–24. https://doi.org/10.1007/s10841-014-9732-7
Allouche O, Kalyuzhny M, Moreno-Rueda G, Pizarro M, Kadmon R (2012) Area–heterogeneity tradeoff and the diversity of ecological communities. PNAS 109:17495–17500. https://doi.org/10.1073/pnas.1208652109
Barton BT (2014) Reduced wind strengthens top-down control of an insect herbivore. Ecology 95:2375–2381. https://doi.org/10.1890/13-2171.1
Barton K (2022) MuMIn: multi-model inference. R Package Version 1.46.6/r495
Bates D, Mächler M, Bolker B, Walker SC (2014) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://arxiv.org/abs/1406.5823
Bergman KO, Daniel-Ferreira J, Milberg P, Ockinger E, Westerberg L (2018) Butterflies in swedish grasslands benefit from forest and respond to landscape composition at different spatial scales. Landsc Ecol 33:2189–2204. https://doi.org/10.1007/s10980-018-0732-y
Bertrand C, Eckerter PW, Ammann L, Entling MH, Gobet E, Herzog F, Albrecht M, Tinner W (2019) Seasonal shifts and complementary use of pollen sources by two bees, a lacewing, and a ladybeetle species in european agricultural landscapes. J Appl Ecol 56:2431–2442. https://doi.org/10.1111/1365-2664.13483
Bjornstad ON, Cai J (2020) Package “ncf”: Spatial Covariance Functions. https://cran.r-project.org/package=ncf
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. TREE 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized Linear mixed modeling. R J 9:378–400 RJ-2017-066.pdf (r-project.org)
Burnham KP, Anderson DR (2001) Model Selection and Inference: A Practical Information-Theoretic Approach. 2nd edition. https://doi.org/10.2307/3803117
Crous CJ, Samways MJ, Pryke JS (2014) Rockiness determines meso-scale conservation of butterflies in afro-montane grassland. J Insect Conserv 18:77–83. https://doi.org/10.1007/s10841-014-9616-x
Dennis RLH, Sparks TH (2005) Landscape resources for the territorial nymphalid butterfly Inachis io: Microsite landform selection and behavioral responses to environmental conditions. J Insect Behav 18:725–742. https://doi.org/10.1007/s/0905-05-7022-7
Dennis RLH, Sparks TH (2006) When is a habitat not a habitat? Dramatic resource use changes under differing weather conditions for the butterfly Plebejus argus. Biol Conserv 129:291–301. https://doi.org/10.1016/j.biocon.2005.10.043
Dennis RLH, Shreeve TG, van Dyck H (2003) Towards a functional resource-based concept for habitat: a butterfly biology perspective. Oikos 102:417–426. http://www.jstor.org/stable/3548046
Dover JW (1989) A method for recording and transcribing observations of butterfly behaviour. Entomol Gaz 40:95–100
Dover JW, Sparks TH, Greatorex-Davies JN (1997) The importance of shelter for butterflies in open landscapes. J Insect Conserv 1:89–97. https://doi.org/10.1023/a:1018487127174
Eckert M, Gaigher R, Pryke JS, Samways MJ (2022) Conservation of complementary habitat types and small-scale spatial heterogeneity enhance soil arthropod diversity. J Environ Manag 317:115482. https://doi.org/10.1016/j.jenvman.2022.115482
Evans LC, Sibly RM, Thorbek P, Sims I, Oliver TH, Walters RJ (2020) The importance of including habitat-specific behaviour in models of butterfly movement. Oecologia 193:249–259. https://doi.org/10.1007/s00442-020-04638-4
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112. https://doi.org/10.1111/j.1461-0248.2010.01559.x
Fartmann T (2006) Oviposition preferences, adjacency of old woodland and isolation explain the distribution of the Duke of Burgundy butterfly (Hamearis lucina) in calcareous grasslands in central Germany. Ann Zool Fenn 43:335–347
Fartmann T, Brüggeshemke J, Poniatowski D, Löffler F (2022) Summer drought affects abundance of grassland grasshoppers differently along an elevation gradient. Ecol Entomol 47:778–790. https://doi.org/10.1111/een.13168
Fischer K, Fiedler K (2001) Resource-based territoriality in the butterfly Lycaena hippothoe and environmentally induced behavioural shifts. Anim Behav 61:723–732. https://doi.org/10.1006/anbe.2000.1662
Gaigher R, Pryke JS, Samways MJ (2019) Divergent fire management leads to multiple beneficial outcomes for butterfly conservation in a production mosaic. J Appl Ecol 56:1322–1332. https://doi.org/10.1111/1365-2664.13357
Gaigher R, Pryke JS, Samways MJ (2021) Habitat complementarity and butterfly traits are essential considerations when mitigating the effects of exotic plantation forestry. Biodivers Conserv 30:4089–4109. https://doi.org/10.1007/s10531-021-02293-6
Habel JC, Angerer V, Gros P, Teucher M, Eberle J (2022) The relevance of transition habitats for butterfly conservation. Biodivers Conserv 31:1577–1590. https://doi.org/10.1007/s10531-022-02411-y
Hartig F (2020) Package “DHARMa”: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. R package version 0.3.0
Harvey JA, Tougeron K, Gols R, Heinen R, Abarca M, Abram PK, Basset Y, Berg M, Boggs C, Brodeur J, Cardoso P, de Boer JG, de Snoo GR, Deacon C, Dell JE, Desneux N, Dillon ME, Duffy GA, Dyer LA, Ellers J, Espíndola A, Fordyce J, Forister ML, Fukushima C, Gage MJD, García-Robledo C, Gely C, Gobbi M, Hallmann C, Hance T, Harte J, Hochkirch A, Hof C, Hoffmann AA, Kingsolver JG, Lamarre GPA, Laurance WF, Lavandero B, Leather SR, Lehmann P, Le Lann C, López-Uribe MM, Ma C, Ma G, Moiroux J, Monticelli L, Nice C, Ode PJ, Pincebourde S, Ripple WJ, Rowe M, Samways MJ, Sentis A, Shah AA, Stork N, Terblanche JS, Thakur MP, Thomas MB, Tylianakis JM, Van Baaren J, Van de Pol M, Van der Putten WH, Van Dyck H, Verberk WCEP, Wagner DL, Weisser WW, Wetzel WC, Woods HA, Wyckhuys KAG, Chown SL (2022) Scientists’ warning on climate change and insects. Ecol Monogr E1553. https://doi.org/10.1002/ecm.1553
Heidrich L, Bae S, Levick S, Seibold S, Weisser W, Krzystek P, Magdon P, Nauss T, Schall P, Serebryanyk A, Wöllauer S, Ammer C, Bässler C, Doerfler I, Fischer M, Gossner MM, Heurich M, Hothorn T, Jung K, Kreft H, Schultze ED, Simons N, Thorn S, Müller J (2020) Heterogeneity–diversity relationships differ between and within trophic levels in temperate forests. Nat Ecol Evol 4:1204–1212. https://doi.org/10.1038/s41559-020-1245-z
Hersbach H, Bell B, Berrisford P, Hirahara S, Horányi A, Muñoz-Sabater J, Nicolas J, Peubey C, Radu R, Schepers D, …, Thépaut J (2020) The ERA5 global reanalysis. Q J R Meteorol Soc 730:1999–2049. https://doi.org/10.1002/qj.3803
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
Hunter ML, Acuña V, Bauer DM, Bell KP, Calhoun AJK, Felipe-Lucia MR, Fitzsimons JA, González E, Kinnison M, Lindenmayer D, Lundquist CJ, Medellin RA, Nelson EJ, Poschlod P (2017) Conserving small natural features with large ecological roles: a synthetic overview. Biol Conserv 211:88–95. https://doi.org/10.1016/j.biocon.2016.12.020
Kadlec T, Tropek R, Konvicka M (2012) Timed surveys and transect walks as comparable methods for monitoring butterflies in small plots. J Insect Conserv 16:275–280. https://doi.org/10.1007/s10841-011-9414-7
Kalarus K, Skórka P, Nowicki P (2013) Resource use in two contrasting habitat types raises different challenges for the conservation of the dryad butterfly Minois dryas. J Insect Conserv 17:777–786. https://doi.org/10.1007/s10841-013-9560-1
Kotze J, Samways MJ (1999) Invertebrate conservation at the interface between the grassland matrix and natural afromontane forest fragments. Biodivers Conserv 8:1339–1363. https://doi.org/10.1023/A
Krauss J, Steffan-Dewenter I, Müller CB, Tscharntke T (2005) Relative importance of resource quantity, isolation and habitat quality for landscape distribution of a monophagous butterfly. Ecography 28:465–474. https://doi.org/10.1111/j.0906-7590.2005.04201.x
Liivamägi A, Kuusemets V, Kaart T, Luig J, Diaz-Forero I (2014) Influence of habitat and landscape on butterfly diversity of semi-natural meadows within forest-dominated landscapes. J Insect Conserv 18:1137–1145. https://doi.org/10.1007/s10841-014-9724-7
Löffler F, Fartmann T (2017) Effects of landscape and habitat quality on Orthoptera assemblages of pre-alpine calcareous grasslands. Agric Ecosyst Environ 248:71–81. https://doi.org/10.1016/j.agee.2017.07.029
Mallinger RE, Gibbs J, Gratton C (2016) Diverse landscapes have a higher abundance and species richness of spring wild bees by providing complementary floral resources over bees’ foraging periods. Landsc Ecol 31:1523–1535. https://doi.org/10.1007/s10980-015-0332-z
Mandelik Y, Winfree R, Neeson T, Kremen C (2012) Complementary habitat use by wild bees in agro-natural landscapes. Ecol Appl 22:1535–1546. https://doi.org/10.1890/11-1299.1
Marini L, Fontana P, Battisti AND (2009) Agricultural management, vegetation traits and landscape drive orthopteran and butterfly diversity in a grassland – forest mosaic: a multi-scale approach. Insect Conserv Divers 2:213–220. https://doi.org/10.1111/j.1752-4598.2009.00053.x
Matenaar D, Bröder L, Bazelet CS, Hochkirch A (2014) Persisting in a windy habitat: Population ecology and behavioral adaptations of two endemic grasshopper species in the Cape region (South Africa). J Insect Conserv 18:447–456. https://doi.org/10.1007/s10841-014-9654-4
Mecenero S, Ball JB, Edge DA, Hamer ML, Henning GA, Kr?ger M, Pringle EL, Terblanche RF, Williams MC (eds) (2013) Conservation assessment of butterflies of South Africa, Lesotho and Swaziland: Red List and atlas. Saftronics (Pty) Ltd., Johannesburg & Animal Demography Unit, Cape Town
Merckx T, van Dyck H (2005) Mate location behaviour of the butterfly pararge aegeria in woodland and fragmented landscapes. Anim Behav 70:411–416. https://doi.org/10.1016/j.anbehav.2004.12.005
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho, and Swaziland. South African National Biodiversity Institute, Pretoria
Mucina L, Scott-shaw C R, Michael C, Camp KGT, Matthews WS, Powrie LW, Hoare DB (2006) Indian Ocean Coastal Belt. Strelizia 19:583
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Öckinger E, Bergman KO, Franzén M, Kadlec T, Krauss J, Kuussaari M, Pöyry J, Smith HG, Steffan-Dewenter I, Bommarco R (2012) The landscape matrix modifies the effect of habitat fragmentation in grassland butterflies. Landsc Ecol 27:121–131. https://doi.org/10.1007/s10980-011-9686-z
Pennington KM, Dickson CGC, Kroon DM (1978) Pennington’s butterflies of Southern Africa. Donker
Pooley E (1993) The Complete Field Guide to Trees of Natal, Zululand and Transkei. Natal Flora Publications Trust, South Africa
Pooley E (1998) A Field Guide to Wildflowers KwaZulu-Natal and the Eastern Region. Natal Flora Publications Trust, South Africa
Pryke SR, Samways MJ (2001) Width of grassland linkages for the conservation of butterflies in south african afforested areas. Biol Conserv 101:85–96. https://doi.org/10.1016/S0006-3207(01)00042-8
Pryke JS, Samways MJ (2012) Conservation management of complex natural forest and plantation edge effects. Landsc Ecol 27:73–85. https://doi.org/10.1007/s10980-011-9668-1
Pryke JS, Vrdoljak SM, Grant PBC, Samways MJ (2012) Butterfly behavioural responses to natural Bornean tropical rain-forest canopy gaps. J Trop Ecol 28:45–54. https://doi.org/10.1017/S0266467411000502
Pryke JS, Roets F, Samways MJ (2013) Importance of habitat heterogeneity in remnant patches for conserving dung beetles. Biodivers Conserv 22:2857–2873. https://doi.org/10.1007/s10531-013-0559-4
R Core Team (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org
Ries L, Fletcher RJ, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Ann Rev Ecol Evol Syst 35:491–522. https://doi.org/10.1146/annurev.ecolsys.35.112202.130148
Samways MJ, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kaila L, Kwak ML, Maes D, Mammola S, Noriega JA, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Settele J, Simaika JP, Stork NE, Suhling F, Vorster C, Cardoso P (2020) Solutions for humanity on how to conserve insects. Biol Conserv 242:e108427. https://doi.org/10.1016/j.biocon.2020.108427
SANBI (2014) Grazing and burning guidelines: managing grasslands for Biodiversity and Livestock Production compiled by RG Lechmere-Oertel. South African National Biodiversity Institute, Pretoria
Schmitt T, Seitz A (2000) Forests as dispersal barriers for Erebia medusa (Nymphalidae, Lepidoptera). Basic Appl Ecol 59:53–59
Schmitt T, Ulrich W, Buschel H, Bretzel J, Gebler J, Mwadime L, Habel J (2020) The relevance of cloud forest fragments and their transition zones for butterfly conservation in Taita Hills, Kenya. Biodivers Conserv 29:3191–3207
Schultz CB, Franco AMA, Crone EE (2012) Response of butterflies to structural and resource boundaries. J Anim Ecol 81:724–734. https://doi.org/10.1111/j.1365-2656.2011.01947.x
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarlı D, Ammer C, Bauhus J, Fischer M, Habel JC, Linsenmair KE, Nauss T, Penone C, Prati D, Schall P, Schulze ED, Vogt J, Wöllauer S, Weisser WW (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674. https://doi.org/10.1038/s41586-019-1684-3
Selwood KE, Zimmer HC (2020) Refuges for biodiversity conservation: a review of the evidence. Biol Conserv 245:e108502. https://doi.org/10.1016/j.biocon.2020.108502
Slamova I, Klecka J, Konvicka M (2013) Woodland and grassland mosaic from a butterfly perspective: Habitat use by Erebia aethiops (Lepidoptera: Satyridae). Insect Conserv Divers 6:243–254. https://doi.org/10.1111/j.1752-4598.2012.00212.x
Stuhldreher G, Fartmann T (2018) Threatened grassland butterflies as indicators of microclimatic niches along an elevational gradient – implications for conservation in times of climate change. Ecol Indic 94:83–98. https://doi.org/10.1016/j.ecolind.2018.06.043
Toivonen M, Peltonen A, Herzon I, Heliölä J, Leikola N, Kuussaari M (2017) High cover of forest increases the abundance of most grassland butterflies in boreal farmland. Insect Conserv Divers 10:321–330. https://doi.org/10.1111/icad.12226
Van Dyck H, Baguette M (2005) Dispersal behaviour in fragmented landscapes: routine or special movements? Basic Appl Ecol 6:535–545. https://doi.org/10.1016/j.baae.2005.03.005
van Halder I, Barbaro L, Jactel H (2011) Conserving butterflies in fragmented plantation forests: are edge and interior habitats equally important? J Insect Conserv 15:591–601. https://doi.org/10.1007/s10841-010-9360-9
van Schalkwyk J, Gaigher R, Pryke JS, Samways MJ (2021) Within-corridor heterogeneity is more important than corridor design for maintaining butterfly functional and taxonomic diversity. J Appl Ecol 58:2734–2746. https://doi.org/10.1111/1365-2664.14006
Wang Y, Naumann U, Wright ST, Warton DI (2012) Mvabund- an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474. https://doi.org/10.1111/j.2041-210X.2012.00190.x
Warton DI, Hui FKC (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10. https://doi.org/10.1890/10-0340.1
Warton DI, Wright ST, Wang Y (2012) Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol 3:89–101. https://doi.org/10.1111/j.2041-210X.2011.00127.x
Woodhall S (2005) Field Guide to butterflies of South Africa. Struik Nature
Yekwayo I, Pryke JS, Roets F, Samways MJ (2016) Surrounding vegetation matters for arthropods of small, natural patches of indigenous forest. Insect Conserv Divers 9:224–235. https://doi.org/10.1111/icad.12160
Yekwayo I, Pryke JS, Roets F, Samways MJ (2017) Responses of ground living arthropods to landscape contrast and context in a forest-grassland mosaic. Biodiv Conserv 26:631–651. https://doi.org/10.1007/s10531-016-1262-z
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
Many thanks to Charl Deacon and Liaam Davids for their assistance in the field and laboratory. We thank Lize Shaw, Gerhard Kruger, Brent Corcoran (Mondi Group), Nerosha Govender (iSimangaliso Wetland Park) and Caroline Fox (Ezemvelo KwaZulu-Natal Wildlife) for logistical support and site access. David Borain (Mondi Group) and Sakeena Allie (EDF-renewables) provided weather data. Arthropod sampling permit: Ezemvelo KwaZulu-Natal Wildlife and iSimangaliso Wetland Park OP 4422/2016.
Funding
The study was funded by the South African National Research Foundation (grant RDYR181213403534) and Mondi Group.
Open access funding provided by Stellenbosch University.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study. R.G. and J.P. collected the data. R.G. analysed the data and wrote the first draft of the manuscript. All authors contributed critically to the manuscript.
Corresponding author
Ethics declarations
Competing interests
James Pryke is co-editor in chief, René Gaigher is an associate editor, and Michael Samways is a patron of the Journal of Insect Conservation. None of the authors here were involved in the reviewing process. The authors have no further competing interests, financial or non-financial, to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gaigher, R., Pryke, J.S. & Samways, M.J. Indigenous forest edges increase habitat complexity and refuge opportunities for grassland butterflies. J Insect Conserv 28, 27–41 (2024). https://doi.org/10.1007/s10841-023-00520-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00520-9