Abstract
We assessed the Caraboidea communities of Gorongosa National Park (GNP) in Mozambique. Influence of tropical rainfall, after a long period of drought, was evaluated on alpha and beta diversity of tiger- and ground-beetles in the main habitat types of the park: miombo forests, mixed forests, transitional forests, and grasslands (open savannas). Tiger- and ground-beetle communities were sampled by pitfall traps set up in 25 sites of each habitat type along three sampling periods, comprising the transition of dry season to the wet season. After the first rainfall, an increase in alpha diversity was observed across GNP habitats, particularly in grasslands. Higher values of beta diversity were observed between the dry and wet sampling periods, particularly in grasslands. In contrast, community dissimilarities between sampling periods were not significant in the transitional forests. Community body size in grasslands increased after the rainfall, partly due to the occurrence of caraboid species that were exclusive of forest habitats during drought. Transitional forests, as ecotone habitat areas, appeared to support grassland species during drought, serving also as a source of forest species that may colonize the open areas in the wet season. Forest species will probably be more threatened by climate aridification and future landscape changes due to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increase in long-lasting periods of drought may affect African landscapes vulnerable to climate aridification, such as the savannas in Mozambique (Engelbrecht et al. 2007; Massad and Castigo 2016; Mbokodo et al. 2020). Even in emblematic protected areas like the Gorongosa Natural Park (GNP), drought is a threat to biodiversity, wildlife and human populations depending on natural resources and ecosystem services (Arndt and Thurlow 2015). These are partly provided by the megafauna of the GNP, but mainly by the “little things that run the world” (Wilson 1987), i.e., the soil invertebrates supporting key ecosystem services (Lavelle et al. 2006). Research studies predicting climate change effects on invertebrate communities need to be built upon reference values of targeted taxonomic groups (Brandmayr and Pizzolotto 2016; Zajicek et al. 2021). Hence, baseline data on species diversity, turnover and seasonal variations are required for future comparisons (Serrano et al. 2023). However, such knowledge is still to be gathered, especially in tropical ecosystems (Brandmayr and Pizzolotto 2016).
Among various functional groups of soil fauna, tiger- and ground-beetles (Coleoptera, Caraboidea) are notable as ecological and biodiversity indicator tools of habitat quality and environmental changes (Rainio and Niemelä 2003; Koivula 2011). They are mostly predators but occupy a large range of trophic levels (Lövei and Sunderland 1996; Kotze et al. 2011), comprising also important prey for several birds, reptiles, and mammals in tropical food webs (Brose 2003). Moreover, tiger- and ground-beetles s are sensitive to climatic changes with temporal patterns reflecting species replacement and turnover among seasons (Kotze et al. 2011; Knapp et al. 2019; Liu et al. 2022). Most species are hygrophilous although there is a wide variation in terms of thermal and moisture tolerance (Kirichenko-Babko et al. 2020; Zajicek et al. 2021). Hence, rainfall patterns shape species compositions among habitats, with highly sensitive species depending more on shelter habitats to outstand climatic extremes (harsh winter or extreme drought) across tropical seasons (Rainio 2013).
Previous authors have discussed the significance of species traits as potential predictors for species sensitivity to environmental changes/stressors, namely body size and dispersal ability, i.e., hind wing morphology (e.g., Kotze and O’Hara 2003; Brooks et al. 2012; Nolte et al. 2017, 2019). Concerning drought, larger-sized beetles may have physiological advantages against desiccation (Entling et al. 2010; Ariza et al. 2021), although smaller species may overcome moisture depletion through behavioral adaptations (e.g., burrowing activity in restricted spaces) minimizing their exposure to drought (Chown and Klok 2003; Homburg et al. 2013). Particularly, small species with long wings, i.e., higher dispersal power, can escape adverse habitat conditions and are usually dominant in disturbed and highly trampled open-habitat types (Blake et al. 1994; Grandchamp et al. 2005; Wang et al. 2018; Ariza et al. 2021). Conversely, large-sized and wingless (or brachypterous) species are more common in closed forest habitats (Jelaska and Durbešić 2009; Wamser et al. 2011; Brandmayr and Pizzolotto 2016) where they find more stable habitat conditions and protection against natural predators (Blake et al. 1994; Brose 2003; Wang et al. 2018). Still, several forest species may use adjacent open areas (e.g., grasslands) as part of their niche, particularly if they find suitable habitat in terms of moisture conditions (Thiele 1977; Holland et al. 2007; Martins da Silva et al. 2009, 2011).
GNP comprises four main habitat types, from miombo and mixed forests to transitional forests and grasslands in the floodplains of lake Urema, although this landscape configuration may suffer changes in the future due to climate change (Corlett 2016; Mbokodo et al. 2020; Massad and Castigo 2016; Herrero et al. 2020). Here we assessed tiger- and ground-beetle communities across GNP habitats, in a sampling period accompanying the transition from the dry to the wet season (first rainfalls). Our hypotheses are that (1) species richness (alpha diversity) will increase after the rainfall, considering that most species strongly depend on the moisture conditions; (2) community turnover (beta diversity) will be higher between the extreme dry and wet sampling perioddue to the tropical rainfall; (3) Grassland habitats will harbor smaller-sized species with higher dispersal power than those from forested habitats and; (4) an increase in body-sized species in grasslands, after the rainfall, will be due to the higher occurrence of beetle species that were confined to the forest habitats in the dry season.
Materials and methods
Study site
Gorongosa National Park (GNP) occupies approximately 4000 km2 of Sofala Province, in central Mozambique, at the southernmost end of Africa’s Great Rift Valley (18°58′04.84″ S, 34°21′41.64″ E) (Stalmans et al. 2019). Annual minimum and maximum temperatures on average range between 15 ◦C and 30 ◦C, in the dry and wet seasons (Herrero et al. 2020). The wet season in Mozambique lasts from October/November to April and mean annual rainfall within the Rift is 700–900 mm. Large areas of the Rift Valley are seasonally flooded after the December–February peak rainfall, resulting in extensive floodplains around Lake Urema in the center of GNP. Rainfall is highly seasonal with a pronounced wet season between December and February (Stalmans et al. 2019).
GNP encompasses large habitat diversity, ranging from grasslands to forests, including the characteristic “miombo”, a closed-canopy savanna dominated by trees of the genus Brachystegia. In the central part of the park, savannas range from “open” savanna (floodplain grassland) to “closed” savannas dominated by several different tree species, i.e., mixed forests and miombo forests, with transitional forests in the ecotone between forested habitats and the floodplain areas near lake Urema (Herrero et al. 2020). About 20% of the valley’s grasslands are flooded much of the year. In this study we focused on biodiversity essays across the main four habitat types of Gorongosa National Park, namely mixed forest, miombo forest, transitional forest and open savanna (grasslands).
Sampling design and beetle sample processing
Field work was carried out in November of 2019 to capture the transition of the dry season to the first rains of the wet season and, at the same time, managing to avoid the flood period, when there is the complete inundation of the habitat types selected for this study.
We selected 25 sampling sites, with 1 km (minimum) between each other, within each habitat type: miombo forest, mixed forest, transitional forest, and open savanna (Serrano et al. 2023). In each sampling site, beetle sampling was carried out using three pitfall traps (sub-samples) disposed in a triangular shape (5 m between pitfalls) in the center of the site. Pitfall traps consisted of plastic vials with 10 cm diameter and filled with ethylene glycol (5%). The traps were covered with a plastic cover (10 cm diameter) fixed a few centimeters aboveground to minimize bycatches of small vertebrates and to prevent flooding and loss of specimens due to heavy rain.
The first rains occurred November 14th (40 mm) with heavy rainfall starting November 20th (110-117 mm), with significantly different levels of precipitation and air humidity in this period in comparison to the precedent dry season (Appendix, Table S1). Thus, to catch the transition between dry to wet sampling periods, the pitfall traps were set during three consecutive sampling periods, lasting ten days each: a dry sampling period preceding the first rains (“Dry”, from 25 October to 5 November), an intermediate sampling period, still in the dry season but experiencing occasional rainfall (“Intermediate”, 5–15 November), and a wet sampling period, including the start of heavy rainfall in Gorongosa (“Wet”: 15–25 November). With this sampling window it was guaranteed that only the wet (and in part the intermediate) sampling period recorded significant weather differences, regarding air humidity and precipitation, then the previous dry season (Appendix, Table S1).
In each sampling site, all pitfall sub-samples were pooled, totalizing 100 samples among habitat types in each sampling period, i.e., ca. 300 samples in all sampling periods. Sampled tiger- and ground-beetles were conserved in pure ethanol and transported to the entomological laboratory at the Centre for Ecology, Evolution and Environmental Changes, in the University of Lisbon (Portugal), for taxonomic identification of all specimens (Serrano et al. 2023). Species identification was supported by several taxonomic references (Serrano et al. 2023), and Caraboidea classification was based on López-López and Vogler (2017). We also collected information on species traits from the literature and/or extracted them directly from the specimens. We selected two species traits, namely average body size (measured in cm) and wing development, i.e., whether they are macropterous (fully winged) or apterous/brachypterous species (e.g., Ribera et al. 2001; Nolte et al. 2017). All data on species taxonomy are presented in Serrano et al. (2023) and species traits are provided in the Appendix (Table S2).
Data analyses
Alpha diversity (abundance) and beta diversity were calculated using R BAT package (Cardoso et al. 2015), implemented in the statistical software R, version 4.1.2 (R Core Team 2021). Beta diversity results were calculated comparing the dry season against sampling periods after the rainfall (intermediate and wet seasons) within each habitat type.
Beta diversity results quantified the degree of temporal turnover from the dry to intermediate and wet season, using a range from 0 to 1. A value of 0 in this range signifies no dissimilarity between the communities being compared: the species composition and abundance in the two communities is identical, and there is complete overlap in the species present. A value of 1 indicates maximum dissimilarity between the communities: there is no overlap in species composition between the communities being compared and they do not share any species in common. Values between 0 and 1 represent intermediate levels of dissimilarity. As the value approaches 1, the dissimilarity between the communities increases, indicating greater turnover of species and a more significant difference in species composition.
To examine the effect of the sampling period on the variation of beetle’ activity-density, alpha and beta diversity across habitat types, we used mixed-models (GLMM), with the habitat as the random factor. For alpha diversity, we employed the Poisson distribution family, and for beta diversity, we used the binomial (link = logit) family. To assess the temporal comparison (DRY1-DRY2 and DRY1-WET) of Beta results, we conducted Wilcoxon tests using the wilcox.test function from the R core package Stats. To assess the habitat influence on activity-density, alpha or beta diversity across seasons we tested the effect of sampling period within each habitat type, performing GLM with the glm function, from the Stats native package from R. The glmmTMB R package (Brooks et al. 2017) was used to perform the tests, and the performance r package (Lüdecke et al. 2021) to obtain R2 values and assess for collinearity between fixed variables.
Associations of most common beetle species (with a minimum of 15 specimens) to specific sampling periods and habitat types were tested by GLM. Beetle’s activity–density data were log(x + 1) transformed to meet the assumptions of normality (Shapiro–Wilk tests) and homoscedasticity (Levene’s tests).
For the whole beetle assemblage, community weighted mean (‘CWM’) of species body size (cm) and dispersal ability (macropterous species – “1”, apterous/brachypterous species – “0”) were compared among habitat types and sampling periods. ‘CWM’ values corresponded to the mean value of a trait in the community, weighted by the relative abundance of the morphotype carrying each trait score (Garnier et al. 2004) and were calculated in the “FD” package (Lalibert et al. 2014). Habitat, sampling period and habitat-season interactions were used as fixed effects in GLM analysis.
Due to the significant differences found in the grasslands, we decided to evaluate whether changes in CWM of body size and dispersal ability in grasslands, along sampling periods, was due to an increase of forest species, by comparing communities in ‘dry grasslands’ vs. ‘wet grasslands with exclusive grassland species’ vs. ‘wet grasslands with exclusive forests species in the dry season’ using GLM analyses.
Fixed effects of all above GLM analyses were tested using the function “anova” performed on the response variables and the fixed factors. For the pairwise comparisons between habitat types (and/or between sampling periods) significant differences were assessed by post-hoc Tukey tests with the “glht” function from the R package “multcomp”, version 1.4–20 (Hothorn et al. 2017).
Results
A total of 1761 specimens from 87 species and 44 genera were collected. In general, a lower absolute number of beetle specimens was collected in the intermediate period and a higher values of activity-density was recorded in the wet sampling (Fig. 1). Values of activity-density varied among habitat types across periods, with a higher proportional number in grasslands and transitional forests in the dry sampling period (Fig. 1). In the wet sampling period, activity-density values were more balanced among habitat types although forested habitat types always recorded a fewer number of individuals (Fig. 1).
Average values of alpha diversity did not vary significantly from dry to the intermediate sampling period, except for the transitional forest habitats (Fig. 2). Higher values of alpha diversity were recorded in the wet sampling period (Table 1), particularly in grassland habitats (Fig. 2). Transitional forest habitats did not clearly follow this general pattern, with no significant differences between dry and wet sampling periods (Table 1). Wilcoxon tests showed that average values of beta diversity were significantly higher between dry and wet sampling period than between dry and intermediate period, especially in the case of grasslands and miombo forest habitats (Fig. 3). This result was only not significant in the case of the transitional habitat type (Fig. 3).
Mean alpha diversity values of tiger- and ground-beetles in the four habitat types (“GRA” – grasslands, “MIX” – mixed forests, “TRA” – transitional forests, “MIO” – miombo forests) along the three sampling periods (“DRY1” – dry period, “DRY2” - intermediate, “WET” – wet period). Each dot represents a sampling site
Beta diversity values between sampling periods (DRY1-DRY2 and DRY1-WET) in the four habitat types (“GRA” – grasslands, “MIX” – mixed forests, “TRA” – transitional forests, “MIO” – miombo forests) using the dry sampling period (“DRY”) as reference. Values range from 0 (the compared sites present the same species and same abundances) to 1 (the compared sites don’t have any species in common). Each dot represents the dissimilarity value of beetle communities in pitfalls between dry - intermediate period (“DRY2”) and dry - wet period (“WET”). Asterisks indicate the significance levels of Wilkoxon tests comparing dissimilarity average values of “DRY2” vs. “WET” (“*” p < 0.05; “**” p < 0.01; “***” p < 0.001)
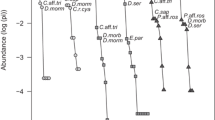
Most common species (more than 80% of total assemblage) showed a clear association to habitat types and sampling periods, i.e., dry or wet (Table 2). Body size values were negatively related to dispersal ability, i.e., larger species are apterous/ brachypterous (F(1,188) = 105.9, P < 0.001; Appendix, Table S3).
CWM of body size was averagely lower in grasslands in relation to the other habitat types and this result was only not significant between grasslands and transitional forests (F(3,190) = 14.71, P < 0.001; Fig. 4). Conversely, the highest CWM of macropterous species was found in grasslands (F = 20.24, P < 0.001; Fig. 5). In this habitat type, CWM of body size increased significantly from dry to wet sampling periods (Fig. 4), while differences in CWM of macropterous species among seasons were not significant (Fig. 5). The increase in community body size within grassland habitats after the rainfall was influenced by the occurrence of forest species in open habitats, e.g., Graphipterus tristis and Anthia alternata, but also the species turnover exclusive species from grasslands along the three sampling periods, e.g., Brachinus distans and Scarites polyphemus (Fig. 6a; Appendix, Table S4).
Community weighted mean of body size (CWM_BS) along the three sampling periods (Dry, Intermediate, Wet) in each habitat type (“GRA” – grasslands, “MIX” – mixed forests, “TRA” – transitional forests, “MIO” – miombo forests); letters “a” and “b” indicate different groups after pairwised Tukey-tests within each habitat type (P < 0.05)
Community weighted mean of dispersal ability (macropterous species – “1”, apterous/brachypterous species – “0”) along the three sampling periods (Dry, Intermediate, Wet) in in each habitat type (“GRA” - grasslands, “MIX” – mixed forests, “TRA” – transitional forests, “MIO” – miombo forests); letters “a” and “b” indicate different groups after pairwised Tukey-tests within each habitat type (P < 0.05)
Community weighted mean of body size (a) and dispersal ability (b) of exclusive grassland species occurring before and after the rainfall (“Dry” and “Exclusive_spp”, respectively), and forest species occurring in grasslands in the wet season (“Forest_spp”); letters “a” and “b” indicate different groups after pairwised Tukey-tests (P < 0.05)
Finally, in transitional forest habitats CWM of body size also increased (Fig. 4), as well as CWM of macropterous species significantly decreased from the dry to the intermediate sampling period (Fig. 5).
Discussion
Rain effects on alpha diversity
Tiger- and ground-beetle’ activity and dynamics are driven by seasonal changes (Lövei and Sunderland 1996; Knapp et al. 2019; Tsafack et al. 2020; Liu et al. 2022) and temporal flooding (den Boer et al. 1986; Lambeets et al. 2008; Kirichenko-Babko et al. 2020). Yet, knowledge is scarce regarding environmental disturbance due to climate change on diversity patterns of tropical ecosystems (Lamarre et al. 2016; Ariza et al. 2021; Peterson et al. 2021). Soil moisture is a key factor influencing site selection for oviposition, successful egg development and survival of soil-dwelling larvae of many carabid species (references in Holland et al. 2007). Hence, they are generally sensitive to soil moisture (e.g., Thiele 1977; Lövei and Sunderland 1996; Rainio and Niemelä 2003; Koivula 2011) and we predicted that rainfall would promote a boost in the number of species after a long period of drought. Our results supported this first hypothesis, as an increase in alpha diversity was generally observed after the heavy rainfall across GNP habitats, particularly in grasslands. This finding was in line with previous studies showing that moisture conditions contribute to increase beetle diversity but depend also on other habitat abiotic and biotic conditions (Luff 1996; Kromp 1999; Brose 2003; Holland et al. 2007; Moret 2009; Kagawa and Maeto 2014; Šustek et al. 2017; Nanni et al. 2019; Peterson et al. 2021). For instance, the combined effect of soil humidity with crop management or cattle grazing intensity, has been reported to benefit ground-beetle diversity either directly or by shaping the plant communities in grassland habitats (Kromp 1999; Martins da Silva et al. 2008, 2011; Waite et al. 2022).
Nevertheless, several other studies have failed to show a significant positive effect of soil moisture on species richness (e.g., Rainio 2013; Schirmel et al. 2014; Lafage and Pétillon 2016; Kirichenko-Babko et al. 2020; Peterson et al. 2021). These findings may be explained by the shift in tiger- and ground-beetle species compositions towards an increase in xerophilous and meso-hygrophilous species, more adapted to drier periods, balancing for the lost in hygrophilous species in the dry season (Holland et al. 2007; Kromp 1999; Martins da Silva et al. 2011; Rainio 2013; Schirmel et al. 2014; Zajicek et al. 2021). Yet, none of the above studies concerned afro-tropical ecosystems. Here we observed that in GNP habitats the lowest values of alpha diversity were not found in the dry season, but in the intermediate season. In the dry sampling period, few species, e.g., Microlestes zambezianus, Microlestes flavipes, Tetragonoderus immaculatus, were more active and clearly dominant, particularly in grasslands and in the transitional forest, which is an ecotone area between forest and grassland habitats. In fact, tiger- and ground-beetle diversity values in transitional forest habitats did not increase significantly after the rainfall, supporting the notion that the effect of moisture depends on the habitat type and configuration. In this case the ecotone forested areas may provide suitable refuge habitats for several xerophilous species which are able to use the adjacent open grasslands for their predatory or herbivorous activity during the dry season (Petit and Usher 1998; Macleod et al. 2004; Ariza et al. 2021). This shelter role of neighbor habitats serving as nesting for many species in harsh environments has been highlighted in previous studies, focusing on agroecosystems and agro-forests (Woodcock et al. 2005; Marasas et al. 2010; Kagawa and Maeto 2014; Martins da Silva et al. 2017; Bennewicz and Barczak 2020).
Rain effects on beta diversity of beetle communities
Previous studies showed that ground-beetle’ community patterns and dynamics are influenced by seasonal changes, determining species turnover due to shifts in thermal and moisture conditions (Boieiro et al. 2013; Williams et al. 2014; Kirichenko-Babko et al. 2020; Hammond et al. 2021; Marrec et al. 2021). Accordingly, and supporting our second hypothesis, we observed that beta diversity was in general strikingly higher between most extremely different seasons, i.e., the dry and wet sampling periods. Habitat changes due to rainfall may promote moisture-dependent species in local communities (e.g., Schirmel et al. 2014) and then determine species turnover among seasons. This result also reflects the harsher conditions during drought for most species, considering that species richness was generally lower in this season in comparison to the wet sampling period. In severely dry environments ground-beetle communities are usually simpler and dominated by a few species more tolerant to drought (e.g., Brandmayr et al. 1983; Tsafack et al. 2020). Still, the survival and activity-density of several species (e.g., M. zambezianus) may be favored by the buffering conditions of shelter habitats, such as canopy and litter cover in forest areas (Marrec et al. 2021; Zajicek et al. 2021; Martins da Silva et al. 2022) or ground slits, shrubs and small tussocks in grasslands (MacLeod et al. 2004; Schirmel et al. 2015; Ariza et al. 2021) during drought. The importance of forest refuge habitats might also explain the lower impact of seasonal rainfall on species turnover in the transitional forest habitats. The buffer role of these ecotone areas between interior forest and open grasslands likely supported the survival and activity of some hygrophilous and xerophilous species (Halme and Niemelä 1993; Heliölä et al. 2001; Yu et al. 2007; Leslie et al. 2014; Boutaud et al. 2022), but particularly of species with broader moisture tolerances (e.g., Pheropsophus mashunus, Pheropsophus insignis) across sampling seasons.
The drought period was especially negative for beetle species in the mixed forest habitat types. In a previous study in GNP (Martins da Silva et al. 2022) we reported mixed forest as the habitat type with higher percentage of canopy cover and this factor may also be related to the higher richness and activity of hygrophilous species in this habitat type. The canopy effect might have served as an environmental buffer against drought during the dry season, since the highest beta diversity in mixed forests was recorded in the intermediate period, instead of the wet sampling period. In fact, most ground-beetles are benefited by moisty environments promoted by thicker litter layers, enabling a higher number of species to locally co-exist (Niemelä 1993; Magura et al. 2018, Magura and Lövei 2019; Marrec et al. 2021; Zajicek et al. 2021). The importance of forest refuge habitats may be confirmed in a future study relating landscape features with caraboid community patterns of GNP.
Species turnover and community traits
Higher activity-density of species with good dispersal power (fully developed wings) was found both in grassland and transitional forest habitats, whereas the species with lower dispersal power were confined to forest habitats during the dry season. Furthermore, in line with our third hypothesis, winged species recorded in grasslands were smaller sized comparing to the wingless species found in forests. Previous studies showed that smaller species with fully developed wings are more adapted to disturbance and have a better dispersal ability to colonize the open habitats (Blake et al. 1994; Grandchamp et al. 2005; Wang et al. 2018) while they may use ecotone habitats as shelter areas (Jelaska and Durbešić 2009; Eyre et al. 2016; Martins da Silva et al. 2017). These eco-morphological traits were also found to favor ground-beetle survival and activity during harsh dry periods in tropical ecosystems (Ariza et al. 2021; Zajicek et al. 2021). Accordingly, we detected a significantly higher number of macropterous species in transitional forest patches during drought. On the opposite side, bigger-sized and wingless species are typically more sensitive to disturbance, depending on forested habitats, particularly if they are moisture-dependent (Halme and Niemelä 1993; Koivula 2002; Jelaska and Durbešić 2009; Wamser et al. 2011; Brandmayr and Pizzolotto 2016; Wang et al. 2018). Larger-sized and wingless species will probably be more threatened by the climate aridification and future landscape changes due to climate change (Kirichenko-Babko et al. 2020; Ariza et al. 2021).
Despite the known habitat preferences of most tiger- and ground-beetles (Thiele 1977; Halme and Niemelä 1993; Niemelä 1993; Koivula 2002; Kotze et al. 2011) there are several generalist’ and ubiquitous species that can extend their home-range to clearcuts or adjacent grasslands, depending on suitable micro-habitat conditions, such as soil humidity, to colonize the open areas (e.g. Heliölä et al. 2001; Brose 2003; Holland et al. 2007; Martins da Silva et al. 2008, 2009, 2011; Litavský et al. 2021). Therefore, we predicted that average body size of beetle communities in grassland would increase in the wet season, reflecting a colonization of grasslands by species that were confined to forest habitats during the dry season. Our results partly supported this hypothesis, since after rainfall a higher community body size was observed in grasslands, partly due to the occurrence of a few species that were exclusive of forest habitats during drought (e.g., Anthia alternata). The average values of community body size in the transitional forest also increased from the dry to the wet season, providing further support for the nesting role of this ecotone habitat for grassland species during drought, serving as a source of forests species that may colonize the open areas (Eversham and Telfer 1994; Desender et al. 2010). However, a few beetle species that appeared in grasslands in the wet season did not occur in forests in the dry sampling season (e.g., Abacetus perturbator, Chlaenius discopictus), indicating that they could be in a dormant stage as eggs, larvae, or pupae during the dry season (Holland et al. 2007; Kotze et al. 2011; Ariza et al. 2021) and were active after the rainfall. In fact, many species may spend a major part of the life cycle in aestivation diapause to cope with harsh low moisture conditions (Kádár et al. 2015; Šiška et al. 2020; Fülöp et al. 2021). Bigger-sized’ specialist species of dry grasslands may be also vulnerable to the predicted longer-lasting periods of drought. Previous authors showed that species associated with extremely dry habitats are at higher risk of extinction (see references in Kotze et al. 2011), especially large carabids of open lands or grasslands, in comparison with large beetles of forests (Kotze and O’Hara 2003; Brandmayr and Pizzolotto 2016). However, these findings were based on Western European ecosystems, reflecting land-use change and agricultural intensification on ground-beetle diversity patterns (e.g., Thiele 1977; Turin and Den Boer 1988; Desender and Turin 1989; Brooks et al. 2012; Nolte et al. 2019). As for the GNP ecosystems, similarly to other tropical ecosystems (e.g., Ariza et al. 2021; Peterson et al. 2021) climate change is expected to be the major driver of tiger- and ground-beetle diversity patterns due to the longer-lasting drought periods and severe flooding events. Considering previous findings on the response of carabid communities to hurricanes/flooding (e.g., Lambeets et al. 2008; Lafage and Pétillon 2016; Litavský et al. 2021) and drought (e.g., Šustek et al. 2017; Šiška et al. 2020; Ariza et al. 2021), in light of our own results, we expect that the increase in these disruptive events and future landscape changes will favor more tolerant (eurytopic) species of smaller size and higher dispersal power, in the savannas of GNP. On the other hand, forest species, more dependent on moisture conditions, will probably be more threatened by climate aridification and future landscape changes due to climate change.
Data Availability
Data used in this study can be assessed through the GBIF link (2022/06/22): Serrano ARM, Carvalho R, Boieiro M, Borges PAV, Martins da Silva P, Baptista M (2023). Inventory of tiger- and ground-beetles (Coleoptera Caraboidea: Cicindelidae, Carabidae) from the Gorongosa National Park (Mozambique). Version 1.8. Universidade dos Açores. Sampling event dataset https://doi.org/10.15468/hwjbhd accessed via GBIF.org on 2023-07-31.
References
Ariza GM, Jácome J, Kotze DJ (2021) Carabid beetles of tropical dry forests display traits that cope with a harsh environment. Int J Trop Insect Sci 41:3011–3021. https://doi.org/10.1007/s42690-021-00493-9
Arndt C, Thurlow J (2015) Climate uncertainty and economic development: evaluating the case of Mozambique to 2050. Clim Change 130:63–75. https://doi.org/10.1007/s10584-014-1294-x
Bennewicz J, Barczak T (2020) Ground beetles (Carabidae) of field margin habitats. Biologia 75:1631–1641. https://doi.org/10.2478/s11756-020-00424-y
Blake S, Foster GN, Eyre MD, Luff ML (1994) Effects of habitat type and grassland management practices on the body size distribution of carabid beetles. Pedobiologia 38:502–512
Boieiro M, Carvalho JC, Cardoso P, Aguiar CAS, Rego C et al (2013) Spatial factors play a major role as determinants of endemic ground beetle beta diversity of Madeira island laurisilva. PLoS ONE 8(5):e64591. https://doi.org/10.1371/journal.pone.0064591
Boutaud E, Nolte D, Harry I (2022) Conservation value of semi-open habitats for ground beetles (Coleoptera: Carabidae, Cicindelidae) in Central Europe. Biodivers Conserv 31:1469–1489. https://doi.org/10.1007/s10531-022-02402-z
Brandmayr P, Pizzolotto R (2016) Climate change impact on carabid beetles. Period biol 118:147–162. https://doi.org/10.18054/pb.2016.118.3.4062
Brandmayr P, Colombetta G, Polli S (1983) Waldcarabiden des Triester Karstes als Indikatoren des makroklimatischen Uebergangs vom kontinentalen Europa zur Mediterraneis. Zool Jb Syst 110:201–220
Brooks DR, Bater JE, Clark SJ et al (2012) Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. J Appl Ecol 49:1009–1019. https://doi.org/10.1111/j.1365-2664.2012.02194.x
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized Linear mixed modeling. RJ 9:378–400. https://doi.org/10.32614/RJ-2017-066
Brose U (2003) Bottom-up control of carabid beetle communities in early successional wetlands: mediated by vegetation structure or plant diversity? Oecologia 135:407–413. https://doi.org/10.1007/s00442-003-1222-7
Cardoso P, Rigal F, Carvalho JC (2015) BAT Biodiversity Assessment Tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol Evol 6:232–236. https://doi.org/10.1111/2041-210X.12310
Chown SL, Klok CJ (2003) Water-balance characteristics respond to changes in body size in subantarctic Weevils. Physiol Biochem Zool 76:634–643. https://doi.org/10.1086/376919
Corlett RT (2016) The impacts of droughts in tropical forests. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2016.02.003. 21:584–593
Den Boer PJ, Luff ML, Mossakowski D, Weber F (1986) Carabid beetles. Their Adaptations and Dynamics. Fischer Verlag, Stuttgart/New York
Desender K, Turin H (1989) Loss of habitat and changes in the composition of the ground and tiger beetles fauna in four west european countries since 1950 (Coleoptera: Carabidae, Cicindelidae). Biol Conserv 48:277–294. https://doi.org/10.1016/0006-3207(89)90103-1
Desender K, Dekoninck W, Dufrêne M, Maes D (2010) Changes in the distribution of carabid beetles in Belgium revisited: have we halted the diversity loss? Biol Conserv 143:1549–1557. https://doi.org/10.1016/j.biocon.2010.03.039
Engelbrecht B, Comita L, Condit R et al (2007) Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447:80–82. https://doi.org/10.1038/nature05747
Entling W, Schmidt-Entling MH, Bacher S, Brandl R, Nentwig W, McGeoch M (2010) Body size—climate relationships of european spiders. J Biogeogr 37:477–485. http://www.jstor.org/stable/25654266
Eversham BC, Telfer MG (1994) Conservation value of roadside verges for stenotopic heathland Carabidae: corridors or refugia? Biodivers Conserv 3:538–545. https://doi.org/10.1007/BF00115159
Eyre MD, McMillan SD, Critchley CNR (2016) Ground beetles. (Coleoptera Carabidae) as indicators of change and pattern in the agroecosystem: longer surveys improve understanding. Ecol Ind 68:82–88. https://doi.org/10.1016/j.ecolind.2015.11.009
Fülöp D, Bérces S, Szabó P et al (2021) Effects of abiotic factors on co-occurring Carabus (Coleoptera: Carabidae) species. Biologia 76:663–671. https://doi.org/10.2478/s11756-020-00593-w
Garnier E, Cortez J, Billes G et al (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637. https://doi.org/10.1890/03-0799
Grandchamp A-C, Bergamini A, Stofer S, Niemelä N, Duelli P, Scheidegger C (2005) The influence of grassland management on ground beetles (Carabidae, Coleoptera) in swiss montane meadows. Agric Ecosyst Environ 110:307–317. https://doi.org/10.1016/j.agee.2005.04.018
Halme E, Niemelä J (1993) Carabid beetles in fragments of coniferous forest. Ann Zool Fenn 30:17–30. https://www.jstor.org/stable/23735353
Hammond HEJ, García-Tejero S, Pohl GR, Langor DW, Spence JR (2021) Spatial and temporal variation of epigaeic beetle assemblages (Coleoptera, Carabidae, Staphylinidae) in aspen-dominated mixedwood forests across north-central Alberta. In: Spence J, Casale A, Assmann T, Liebherr JК, Penev L (eds) Systematic Zoology and Biodiversity Science: A tribute to Terry Erwin (1940–2020). ZooKeys 1044:951–991. https://doi.org/10.3897/zookeys.1044.65776
Heliölä J, Koivula M, Niemelä J (2001) Distribution of carabid beetles (Coleoptera, Carabidae) across boreal forest—clear-cut ecotone. Conserv Biol 15:370–377. https://doi.org/10.1046/j.1523-1739.2001.015002370.x
Herrero H, Waylen P, Southworth J, Khatami R, Yang D, Child B (2020) A healthy park needs healthy vegetation: the story of Gorongosa National Park in the 21st century. Remote Sens 12:476. https://doi.org/10.3390/rs12030476
Holland JM, Thomas CFG, Birkett T, Southway S (2007) Spatio-temporal distribution and emergence of beetles in arable fields in relation to soil moisture. Agric Ecosyst Environ 97:89–100. https://doi.org/10.1017/S0007485307004804
Homburg K, Schuldt A, Drees C, Assmann T (2013) Broad-scale geographic patterns in body size and hind wing development of western Palaearctic carabid beetles (Coleoptera: Carabidae). Ecography 36:166–177. https://doi.org/10.1111/j.1600-0587.2012.07488.x
Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A, Scheibe S (2017) Simultaneous inference in general parametric models. R package ‘multcomp’, Version 1.4–20. http://multcomp.R-forge.R-project.org
Jelaska L, Durbešić P (2009) Comparison of the body size and wing form of carabid species (Coleoptera: Carabidae) between isolated and continuous forest habitats. Ann Soc Entomol Fr 45:327–338. https://doi.org/10.1080/00379271.2009.10697618
Kádár F, Fazekas JP, Sárospataki M, Lövei G (2015) Seasonal dynamics, age structure and reproduction of four Carabus species (Coleoptera: Carabidae) living in forested landscapes in Hungary. Acta Zool Acad Sci Hung 61:57–72. https://doi.org/10.17109/AZH.61.1.57.2015
Kagawa Y, Maeto K (2014) Ground beetle (Coleoptera: Carabidae) assemblages associated with a satoyama landscape in Japan: the effects of soil moisture, weed height, and distance from woodlands. Appl Entomol Zool 49:429–436. https://doi.org/10.1007/s13355-014-0266-y
Kirichenko-Babko M, Danko Y, Musz-Pomorksa A, Widomski MK, Babko R (2020) The impact of climate variations on the structure of ground beetle (Coleoptera: Carabidae) assemblage in forests and wetlands. Forests 11:1074. https://doi.org/10.3390/f11101074
Knapp M, Seidl M, Knappová J et al (2019) Temporal changes in the spatial distribution of carabid beetles around arable field-woodlot boundaries. Sci Rep 9:8967. https://doi.org/10.1038/s41598-019-45378-7
Koivula M (2002) Alternative harvesting methods and boreal carabid beetles (Coleoptera, Carabidae). For Ecol Manag 167:103–121. https://doi.org/10.1016/S0378-1127(01)00717-4
Koivula MJ (2011) Useful model organisms, indicators, or both? Ground beetles (Coleoptera, Carabidae) reflecting environmental conditions. In: Kotze DJ, Assmann T, Noordijk J, Turin H, Vermeulen R (eds) Carabid Beetles as Bioindicators: Biogeographical, Ecological and Environmental Studies. ZooKeys 100:287–317. https://doi.org/10.3897/zookeys.100.1533
Kotze DJ, O’Hara RB (2003) Species decline—but why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 135:138–148. https://doi.org/10.1007/s00442-002-1174-3
Kotze DJ, Brandmayr P, Casale A et al (2011) Forty years of carabid beetle research in Europe – from taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. ZooKeys 100:55–148. https://doi.org/10.3897/zookeys.100.1523
Kromp B (1999) Carabid beetles in sustainable agriculture: a review on pest control efficacy, cultivation impacts and enhancement. Agric Ecosyst Environ 74:187–228. https://doi.org/10.1016/S0167-8809(99)00037-7
Lafage D, Pétillon J (2016) Relative importance of management and natural flooding on spider, carabid and plant assemblages in extensively used grasslands along the Loire. Basic Appl Ecol. 17:535–545. https://doi.org/10.1016/j.baae.2016.04.002
Laliberté E, Legendre P, Shipley B (2014) Package ‘FD’. Measuring functional diversity (FD) from multiple traits, and other tools for functional ecology. R package
Lamarre GPA, Hérault B, Fine PVA, Vedel V, Lupoli R, Mesones I, Baraloto C (2016) Taxonomic and functional composition of arthropod assemblages across contrasting amazonian forests. J Anim Ecol 85:227–239. https://doi.org/10.1111/1365-2656.12445
Lambeets K, Vandegehuchte ML, Maelfait J-P, Bonte D (2008) Understanding the impact of flooding on trait-displacements and shifts in assemblage structure of Predatory Arthropods on River Banks. J Anim Ecol 77:1162–1174. http://www.jstor.org/stable/20143299
Lavelle P, Decaëns T, Aubert M, Barot S, Blouin M, Bureau F, Margerie P, Mora P, Rossi J-P (2006) Soil invertebrates and ecosystem services. Eur J Soil Biol 42:3–15. https://doi.org/10.1016/j.ejsobi.2006.10.002
Leslie TW, Biddinger DJ, Rohr JR, Hulting AG, Mortensen DA, Fleischer SJ (2014) Examining shifts in Carabidae assemblages across a forest-agriculture ecotone. Environ Entomol 43:18–28. https://doi.org/10.1603/EN13099
Litavský J, Majzlan O, StaTAšiov S, Svitok M, Fedor P (2021) The associations between ground beetle (Coleoptera: Carabidae) communities and environmental condition in floodplain forests in the Pannonian Basin. Eur J Entomol 118:14–23. https://doi.org/10.14411/eje.2021.002
Liu S, Dong S, Liu R et al (2022) The distribution patterns and temporal dynamics of carabid beetles (Coleoptera: Carabidae) in the forests of Jiaohe, Jilin Province, China. J for Res 33:333–342. https://doi.org/10.1007/s11676-021-01367-z
López-López A, Vogler AP (2017) The mitogenome phylogeny of Adephaga (Coleoptera). Mol Phylogenet Evol 114:166–174. https://doi.org/10.1016/j.ympev.2017.06.009
Lövei GL, Sunderland KD (1996) Ecology and Behavior of Ground Beetles (Coleoptera: Carabidae). Annu Rev Entomol. 41:231–256. https://doi.org/10.1146/annurev.en.41.010196.001311
Lüdecke D, Ben-Shachar M, Patil I, Waggoner P, Makowski D (2021) PERFORMANCE: an R Package for assessment, comparison and testing of statistical models. J Open Source Softw 6(60). https://doi.org/10.21105/joss.03139
Luff ML (1996) Use of carabids as environmental indicators in grasslands and cereals. Ann Zool Fenn 33:185–195. https://www.jstor.org/stable/23735418
MacLeod A, Wratten SD, Sotherton NW, Thomas MB (2004) Beetle banks’ as refuges for beneficial arthropods in farmland: long-term changes in predator communities and habitat. Agr for Entomol 6:147–154. https://doi.org/10.1111/j.1461-9563.2004.00215.x
Magura T, Lövei GL (2019) Environmental filtering is the main assembly rule of ground beetles in the forest and its edge but not in the adjacent grassland. Insect Sci 26:154–163. https://doi.org/10.1111/1744-7917.12504
Magura T, Lövei GL, Tóthmérész B (2018) Conversion from environmental filtering to randomness as assembly rule of ground beetle assemblages along an urbanization gradient. Sci Rep 8:16992. https://doi.org/10.1038/s41598-018-35293-8
Marasas ME, Sarandón SJ, Cicchino A (2010) Semi-Natural Habitats and Field margins in a typical agroecosystem of the Argentinean Pampas as a Reservoir of Carabid Beetles. J Sustain Agric 34:153–168. https://doi.org/10.1080/10440040903482563
Marrec R, Le Roux V, Martin L et al (2021) Multiscale drivers of carabid beetle (Coleoptera: Carabidae) assemblages in small european woodlands. Global Ecol Biogeogr 30:165–182. https://doi.org/10.1111/geb.13208
Martins da Silva P, Aguiar CAS, Niemelä J, Sousa JP, Serrano ARM (2008) Diversity patterns of ground-beetles (Coleoptera: Carabidae) along a gradient of land-use disturbance. Agric Ecosyst Environ 124:270–274. https://doi.org/10.1016/j.agee.2007.10.007
Martins da Silva P, Aguiar CAS, Niemelä J, Sousa JP, Serrano ARM (2009) Cork-oak woodlands as key-habitats for biodiversity conservation in Mediterranean landscapes: a case study using rove and ground beetles (Coleoptera: Staphylinidae, Carabidae). Biodivers Conserv 18:605–619. https://doi.org/10.1007/s10531-008-9527-9
Martins da Silva P, Aguiar CAS, de Faria e Silva I, Serrano ARM (2011) Orchard and riparian habitats enhance ground dwelling beetle diversity in Mediterranean agro-forestry systems. Biodivers Conserv 20:861–872. https://doi.org/10.1007/s10531-010-9987-6
Martins da Silva P, Oliveira J, Ferreira A, Fonseca F, Pereira JA, Aguiar CA, Serrano ARM, Sousa JP, Santos SA (2017) Habitat structure and neighbor linear features influence more carabid functional diversity in olive groves than the farming system. Ecol Ind 79:128–138. https://doi.org/10.1016/j.ecolind.2017.04.022
Martins da Silva P, Bartz M, Mendes S, Boieiro M, Timóteo S, Azevedo-Pereira HMVS, Alves da Silva A, Alves J, Serrano ARM, Sousa JP (2022) Tree Canopy enhances Collembola Functional Richness and Diversity Across typical habitats of the Gorongosa National Park (Mozambique). Appl Soil Ecol (under review). https://doi.org/10.2139/ssrn.4055105
Massad TJ, Castigo T (2016) Investigating possible effects of climate change on tree recruitment: responses of abundant species to water stress in Gorongosa National Park. Afr S J Bot 106:96–100. https://doi.org/10.1016/j.sajb.2016.06.002
Mbokodo I, Bopape M-J, Chikoore H, Engelbrecht F, Nethengwe N (2020) Heatwaves in the Future Warmer Climate of South Africa. Atmosphere 11:712. https://doi.org/10.3390/atmos11070712
Moret P (2009) Altitudinal distribution, diversity and endemicity of Carabidae (Coleoptera) in the páramos of ecuadorian Andes. Ann Soc Entomol Fr. 45:500–510. https://doi.org/10.1080/00379271.2009.10697632
Nanni AS, Fracassi NG, Magnano AL et al (2019) Ground Beetles in a changing world: Communities in a modified Wetland Landscape. Neotrop Entomol 48:729–738. https://doi.org/10.1007/s13744-019-00689-2
Niemelä J (1993) Interspecific competition in ground-beetle assemblages (Carabidae): what have we learned? Oikos 66:325–335. https://doi.org/10.2307/3544821
Nolte D, Schuldt A, Gossner MM, Ulrich W, Assmann T (2017) Functional traits drive ground beetle community structures in central european forests: implications for conservation. Biol Conserv 213:5–12. https://doi.org/10.1016/j.biocon.2017.06.038
Nolte D, Boutaud E, Kotze DJ, Schuldt A, Assmann T (2019) Habitat specialization, distribution range size and body size drive extinction risk in carabid beetles. Biodivers Conserv. 28:1267–1283. https://doi.org/10.1007/s10531-019-01724-9
Peterson KNR, Browne RA, Erwin TL (2021) Carabid beetle (Coleoptera, Carabidae) richness, diversity, and community structure in the understory of temporarily flooded and non-flooded Amazonian forests of Ecuador. In: Spence J, Casale A, Assmann T, Liebherr JK, Penev L (eds) Systematic Zoology and Biodiversity Science: A tribute to Terry Erwin (1940–2020). ZooKeys 1044:831–876. https://doi.org/10.3897/zookeys.1044.62340
Petit S, Usher MB (1998) Biodiversity in agricultural landscapes: the ground beetle communities of Woody uncultivated habitats. Biodivers Conserv 7:1549–1561. https://doi.org/10.1023/A:1008875403868
R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Rainio J (2013) Seasonal Variation of Carabid Beetle (Coleoptera: Carabidae) abundance and diversity in Ranomafana National Park, Madagascar. J Entomol Zool Stud 1:92–98
Rainio J, Niemelä J (2003) Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers Conserv 12:487–506. https://doi.org/10.1023/A:1022412617568
Ribera I, Dolédec S, Downie IS, Foster GN (2001) Effect of land disturbance and stress on species Traits of ground beetle assemblages. Ecology 82:1112–1129. https://doi.org/10.1890/0012-9658(2001)082[1112:EOLDAS]2.0.CO;2
Schirmel J, Alt M, Rudolph I, Entling MH (2014) Effects of Traditional Flood Irrigation on Invertebrates in Lowland Meadows. PLoS ONE 9(10):e110854. https://doi.org/10.1371/journal.pone.0110854
Schirmel J, Mantilla-Contreras J, Gauger D, Blindow I (2015) Carabid beetles as indicators for shrub encroachment in dry grasslands. Ecol Ind 49:76–82. https://doi.org/10.1016/j.ecolind.2014.09.041
Serrano ARM, Baptista M, Carvalho R, Boieiro M, Mendes S, Bartz M, Timóteo S, Azevedo-Pereira HMVS, Aguiar CAS, Alves da Silva A, Alves J, Briones MJ, Sousa JP, da Martins P (2023) Inventory of tiger- and ground-beetles (Coleoptera Caraboidea: Cicindelidae, Carabidae) in two sampling seasons of the Gorongosa National Park. Biodivers Data J (in press)
Šiška B, Eliašová M, Kollár J (2020) Carabus Population Response to Drought in Lowland Oak Hornbeam Forest. Water 12:3284. https://doi.org/10.3390/w12113284
Stalmans ME, Massad TJ, Peel MJS, Tarnita CE, Pringle RM (2019) War-induced collapse and asymmetric recovery of large-mammal populations in Gorongosa National Park, Mozambique. PLoS ONE 14:e0212864. https://doi.org/10.1371/journal.pone.0212864
Šustek Z, Vido J, Škvareninová J, Škvarenina J, Šurda P (2017) Drought impact on ground beetle assemblages (Coleoptera, Carabidae) in Norway spruce forests with different management after windstorm damage – a case study from Tatra Mts. (Slovakia). J Hydrol Hydromech 65:333–342. https://doi.org/10.1515/johh-2017-0048
Thiele HU (1977) Carabid beetles in their environments. Springer, Berlin Heidelberg New York
Tsafack N, Xie Y, Wang X, Fattorini S (2020) Influence of climate and local habitat characteristics on carabid beetle abundance and diversity in Northern Chinese steppes. Insects 11:19. https://doi.org/10.3390/insects11010019
Turin H, Den Boer PJ (1988) Changes in the distribution of carabid beetles in the Netherlands since 1880. II. Isolation of habitats and long-term time trends in the occurrence of carabid species with different powers of dispersal (Coleoptera, Carabidae). Biol Conserv 44:179–200. https://doi.org/10.1016/0006-3207(88)90101-2
Waite ES, Houseman GR, Jensen WE, Reichenborn MM, Jameson ML (2022) Ground Beetle (Coleoptera: Carabidae) responses to cattle grazing, grassland restoration, and habitat across a precipitation gradient. Insects 13:696. https://doi.org/10.3390/insects13080696
Wamser S, Dauber J, Birkhofer K, Wolters V (2011) Delayed colonisation of arable fields by spring breeding ground beetles (Coleoptera: Carabidae) in landscapes with a high availability of hibernation sites. Agric Ecosyst Environ 144:235–240. https://doi.org/10.1016/j.agee.2011.08.019
Wang X, Steiner M, Schütz M, Vandegehuchte ML, Risch AC (2018) Progressively excluding mammals of different body size affects community and trait structure of ground beetles. Oikos 127:1515–1525. https://doi.org/10.1111/oik.05198
Williams RS, Marbert BS, Fisk MC, Hanson PJ (2014) Ground-dwelling beetle responses to long-term precipitation alterations in a hardwood forest. Southeast Nat 13:138–155. http://www.jstor.org/stable/26454381
Wilson EO (1987) The little things that run the world (the importance and conservation of invertebrates). Conserv Biol 1:344–346. http://www.jstor.org/stable/2386020?origin=JSTOR-pdf
Woodcock BA, Westbury DB, Potts SG, Harris SJ, Brown VK (2005) Establishing field margins to promote beetle conservation in arable farms. Agric Ecosyst Environ 107:255–266. https://doi.org/10.1016/j.agee.2004.10.029
Yu XD, Luo TH, Zhou HZ, Yang J (2007) Distribution of carabid beetles (Coleoptera: Carabidae) across a forest-grassland ecotone in southwestern China. Environ Entomol. 36:348–55. https://doi.org/10.1603/0046-225x(2007)36[348:docbcc]2.0.co;2
Zajicek P, Welti EAR, Baker NJ et al (2021) Long-term data reveal unimodal responses of ground beetle abundance to precipitation and land use but no changes in taxonomic and functional diversity. Sci Rep 11:17468. https://doi.org/10.1038/s41598-021-96910-7
Acknowledgements
This study was supported by the Project ECOASSESS – A biodiveristy and ECOlogical ASSESSment of soil fauna of Gorongosa National Park (Mozambique) (PTDC/BIA¬CBI/29672/2017) funded through national funds by FCT / MCTES (PIDDAC) under the Programme All Scientific Domains. Pedro Martins da Silva and Mário Boieiro were supported by FCT under contracts DL57/2016/IT057-18-7285 and DL57/2016/CP1375/CT0001, respectively. Marie Bartz, Sara Mendes, Sérgio Timóteo, Henrique Pereira and Maria Briones carried out the field sampling with the logistic support of Gorongosa National Park under supervision of Jason Denlinger (Lab manager) and Mark Stalmans (Director of Scientific Service). Carlos A.S. Aguiar, Martim Baptista and Sara Mendes sorted Caraboidea specimens from the collected samples. We are also grateful to João Malato for helping with the data analyses.
Funding
This study was supported by the Project ECOASSESS – A biodiveristy and ECOlogical ASSESSment of soil fauna of Gorongosa National Park (Mozambique) (PTDC/BIA¬CBI/29672/2017) funded through national funds by FCT / MCTES (PIDDAC) under the Programme All Scientific Domains.
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
PMS wrote the main manuscript text. RC, MB and ARMS contributed to the writing of the manuscript. PMS, RC, MB, ARMS and JPS contributed to the concept and design. RC and PMS analyzed the data. RC prepared Figs. 1, 2 and 3 and PMS prepared Figs. 4, 5, 6 and 7. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martins da Silva, P., Carvalho, R., Boieiro, M. et al. Long droughts decrease tiger- and ground-beetle’ beta diversity and community body size in savannas of the Gorongosa National Park (Mozambique). J Insect Conserv 27, 927–940 (2023). https://doi.org/10.1007/s10841-023-00509-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00509-4