Abstract
While some species rapidly decline, compromising vital ecosystem services, others are stable or even increasing in abundance. We studied shifts in species richness and abundance of moths over five decades in Central Sweden and examined whether and how changes were associated with phenotypic traits. We combined information from captures in a light trap in historical time (the 1970s) and contemporarily (2019) with provincial colonisation records. Both community species richness and the abundance of moths increased between the time periods. A large fraction (38%) of the 410 species were not present in both periods, 101 species had colonised the area (an estimated influx of about 2.5 new species per year), and 51 species had disappeared (an estimated local extinction rate of about 1.16 species per year). Abundance increased with an increasing number of host plants and with increasing colour pattern variation. The composition of the community shifted towards species characterised by smaller wingspan and larger ranges. The functional trait variability of the moth community was homogenised from the historical to the contemporary period, in line with reports from other species communities in modern landscapes. However, our finding of a concomitant increase in species richness and total abundance differs from the results of many recent studies of insect communities in other geographic regions.
Implication for insect conservation
We conclude that the entire boreal moth fauna is undergoing a substantial biogeographical shift, changing to the moth species historically occurring at more southern latitudes. The alterations in species composition, overall abundance, and community-wide trait distribution described here may cascade up and down in the ecosystem because moths are important pollinators, herbivores, and prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We are facing the sixth mass extinction (Dirzo et al. 2014). While many populations are rapidly declining, compromising vital ecosystem services, others are stable or even increasing (Antão et al. 2020; Crossley et al. 2021; Macgregor et al. 2019). Insects are the most species-rich group of animals and provide important ecosystem services (e.g., pollination) and disservices (e.g. crop and forest damage, disease transmission) (Gaston 1991). Recent reports of declines in abundance, distribution, and species richness of insect communities worldwide are alarming and a cause of great concern (van Klink et al. 2020), and yet it is still debated whether claims of catastrophic collapse of the insect fauna (Crossley et al. 2020; Lister and Garcia 2018; Wagner et al. 2021) are justified (Schowalter et al. 2021; Thomas et al. 2019). Some studies report that insects fluctuate greatly over time with halting losses observed in recent decades (Carvalheiro et al. 2013), lack of any clear trends (Crossley et al. 2020), and even tendencies that species richness and abundance have increased, such as in northern UK, Finland, and parts of North America (Antão et al. 2020; Macgregor et al. 2019; Crossley et al. 2021). The above variability and inconsistency of outcomes point to context-specific responses to environmental change. For example, in temperate areas, where historical factors limit species distributions, there should be ample opportunities for northward range expansions and increased abundances following climate change, reviewed in Forsman et al. (2016).
Long-term persistence and successful establishments in new areas require suitable environmental conditions(Parmesan and Yohe 2003; Pöyry et al. 2016). Previous studies of moths suggest that, in general, species that occupy large geographic ranges and are characterised by a generalist lifestyle, high mobility, and highly variable colour patterns are better able to cope with changing environments, colonise new habitats, and avoid local extinction (Forsman et al. 2020; Franzen and Johannesson 2007; Nilsson et al. 2008). Conversely, specialised species are generally believed to suffer most from environmental change. Still, under certain circumstances, specialists can also take advantage of emerging environmental conditions. For example, it is advantageous to specialise in resources favoured by soil eutrophication (Betzholtz et al. 2013). Indeed, evidence is mounting that, depending on the combination of trait values, some species can cope relatively well with current rates of environmental changes while others can not (Ceballos et al. 2020; Parmesan and Yohe 2003), but see (Crossley et al. 2021). The resulting loss of specific species traits and more homogeneous communities implies a higher vulnerability to changes and less functional ecosystems, as has been shown in grassland (Gossner et al. 2016), forest (Gilliam 2006), and coral reefs fish communities (Richardson et al. 2018).
There has yet to be a consensus regarding how species and communities respond to current environmental changes. Each contribution that informs about temporal shifts in species richness, distribution, and abundance is critically important. This study combines information from captures in a light trap with new provincial records (the year a species is found for the first time in the province Gästrikland) to examine how species richness, species composition, total abundance, and community-wide trait distributions of moth communities in central Sweden have changed over five decades (from the 1970s to 2019). Specifically, we address the following four questions:
-
(i)
How has species richness changed over the past five decades, how many species have colonised the area, how many species have faced local extinction, and how many species have persisted since the 1970s?
-
(ii)
Is the variation in abundance among species in the historical period associated with the variation in abundance in the contemporary time period?
-
(iii)
How are colonisations, local extinctions, and abundance shifts associated with the variation among species in phenotypic traits (colour pattern, diet, habitat use, wingspan, and range size)?
-
(iv)
How have the temporal shifts in species richness, species composition, and abundance influenced the community-wide trait distribution?
Materials and methods
Study area
The data collected in the 1970s are hereafter called the historical period, and the data collected in 2019 are hereafter called the contemporary period. To compare historical and contemporary moth communities, we explored information in the province records and yearly species reports for the province Gästrikland in Sweden (60N, 17E) and information on species records from trapping data from one light trap that was in operation during the full season and checked every night during both periods at the same place in this province. Gästrikland covers an area of 4181 km² and has 37.6 inhabitants per km².
The climate in the region is temperate, with cold temperatures and high precipitation. The yearly precipitation is 620 mm, and the average yearly temperature extracted from the Gävle weather station is 5.82 ∘C from 1874 to 2019 (SMHI, online resource, Fig. 1). The light trap was situated 8 km northwest of the city of Gävle in central Sweden (60.751N, 17.108E). The light trap was placed in an abandoned garden, presently used as a semi-dry hay meadow, surrounded by scattered bedrocks and mixed forests dominated by pine and spruce. The area is dominated by forests, arable fields, and gardens in 2019 (Fig. S1). To visually illustrate the landscape surrounding the light trap, we used aerial photographs from 1961 to 1975 and SPOT-5 satellite images from 2005 to 2018 (downloaded from lantmateriet.se, Fig. S1). This shows that in 1961 much of the forest had fallen due to a storm in January 1954. Also, some arable fields disappeared, and buildings increased from 1961 to 2018 (Fig. S1). The trap site and the province is representative of the landscape and the changes that have occurred throughout this region in general (Anonymous 1996, 2020).
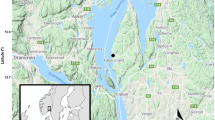
Example of studied species, study area, and between year variation in ambient temperature. a Orthosia gothica is the species with the highest increase in abundance, a variable colour pattern, and a habitat and diet generalist. b Entephria caesiata, an extinct species that show dramatic decline throughout the boreal region, is a habitat specialist associated with coniferous forests and has a few host plants (oligolectic). c Catocala nupta is an expanding species that colonized the area and is associated with forests and a few host plants (oligolectic)—photographs by Vladimir S. Kononenko. d The province Gästrikland in central Sweden in red. e The province Gästrikland in red and the light trap site denoted by a red dot. f Temperature anomalies in Gävle from 1874 to 2020 with the sampled years indicated by green triangles
Collection of data on species presence, abundance, establishment, and local extinction
Based on province records, the year each species colonised the province Gästrikland was extracted from province catalogues and yearly reports (Betzholtz et al. 2013; Douwes 1974; Nordström 1943). There is a long-lasting tradition to record new province records in Sweden, and thus it captures colonising species over time. The first catalogue with a good cover was established in 1943 and contained records from 1936 (Nordström 1943) and 1973, and from thereon, yearly reports have been published (Douwes 1974). New province records most likely represent true natural range expansions and establishments in the province, rather than the outcome of intensified sampling and detection of species that have been present but remained undetected for long periods, as the province has been well investigated since 1936 (Nordström 1952).
In addition to the province records, we used data on moths for both periods collected by one of the authors (Sjöberg) using a light trap (Leinonen et al. 1998). The light trap was operating at the same position during the whole moth season covering 226 nights (April 9–November 20) in both the historical and the contemporary periods. We compiled a series of full-season sampling by combining trapping nights in 1975 (131 nights from April to November), 1976 (13 nights in July), 1977 (42 nights from April to August), and 1978 (25 nights in July and August). We had to combine data from multiple years in the 1970s to reach a fully sampled season to justify a comparison with the fully sampled season in 2019. Moths were present in 156 and 189 trapping nights in the historical and contemporary periods, suggesting that nights with zero counts were more frequent in the historical period. Information on individual counts per species per night is available for all nights except for August 13 to August 26 in the historical period. For these 2 weeks, information on the total catch only is available. This limited period re-distributed the total catch among the 14 trapping nights between August 13 and August 26. The trap was equipped with a 400 W mercury bulb in the historical period and a 125 W mercury bulb in the contemporary period. All individuals were identified to species, counted, and recorded on a nightly basis. The lamp was automatically switched on at dawn and off at dusk using twilight sensors. In the historical period, a white sheet was manually checked throughout each night. In the contemporary period, individuals were sampled from a trap filled with egg cartons. The different bulbs and sheet vs. trap design that differed between the two periods are unlikely to influence the results as the lamps, sheet and trap are similar in their efficiency (Leinonen et al. 1998). We used slightly different approaches as the lamp and trap were not invented in the 1970s and is now standard and less labour intensive compared to the method used in the 1970s in moth monitoring programmes. To test and evaluate the efficiency of the sampling in the historical and contemporary period we used a rarefaction approach (see below). One of the authors (Sjöberg) identified, counted, and registered all traditionally classified species as Macrolepidoptera (Appendix 1). Taxonomically complex taxa were pooled (n = 7), and the genus Eupithecia was grouped into three taxa (Appendix 1). Identifications were verified and standardised to current taxonomic knowledge (Aarvik et al. 2017). Each record consisted of a species name, number of individuals, and the date of capture. Except for the analysis of rarefaction and comparisons of nightly trap catches that are based on nightly counts we calculate the total abundance for each species and period for the statistical analyses by summing the captures across all trap nights.
Extraction of information on species traits
We extracted data on colour pattern variation, diet specialisation, habitat preference, wingspan, and range size (Appendix 2). These five species traits were chosen because previous studies indicate that they are indicative of a specialist-generalistic lifestyle, and may influence species-area requirements, the rate of range expansions, abundance trends over time, and extinction risk in moths (Betzholtz et al. 2017; Forsman et al. 2016, 2020; Franzén et al. 2012).
Statistical analyses
Data were analysed using R version 4.0.3 (R Core Team 2021).
Estimating species richness and abundance
To examine if our light-trap detected most species, we evaluated the sampling intensity in the historical and contemporary periods. We used a rarefaction approach for this purpose and the specaccum function from the vegan package (version 4.0.3) (Oksanen et al. 2019) to compute rarefaction-based species accumulation curves and standard error. This estimator was chosen because it is widely used and evaluated in the ecological literature (Chao et al. 2005, Coleman et al. 1982, Hellmann and Fowler 1999, Reese et al. 2014). To test whether nightly abundance and species richness of moths were statistically different between the historical and contemporary periods we used Mann–Whitney–Wilcoxon test. The test was based on only the matching days per year in the 1970s, sampled days in 1975 vs. the identical days in 2019, sampled days in 1976 vs. the identical days in 2019, and the same procedure for 1977 and 1978.
To test whether abundance in the historical period is related to the abundance in the contemporary period, we performed Pearson correlation analysis based on log-transformed (abundance + 1) values. To test whether the average abundance changed between the historical and contemporary period, whether abundance changes varied among species, and whether the variation in abundance changes among species was associated with species traits, we built a general linear mixed model (GLMM) with Gaussian distribution in the package glmmTMB (version 1.1.1) (Brooks et al. 2017). The difference in observed abundance between the contemporary and historical periods was the response variable and the five traits (colour pattern variation, continuous; diet specialisation categorical with three levels; habitat preference, categorical with three levels; wingspan, continuous; and range size, continuous) as fixed effects and the taxonomic group as a random effect to partially account for variation owing to shared ancestry and phylogenetic relatedness. With this analytical approach, colonisations and local extinctions contribute to the variation in abundance shifts between the two time periods. We were interested in evaluating whether changes in abundance between periods were associated with species traits. Model selection was based on minimising the Akaike Information Criterion (AIC) (Burnham, 2002). Model marginal effects and confidence intervals (CIs) were calculated using the ggeffects package (version 1.1.0) (Lüdecke 2018).
Analysing and comparing community-wide trait distributions
To illustrate, describe and compare the trait composition that characterises moth communities in the historical versus the contemporary period, we used nonmetric multidimensional scaling (NMDS). In NMDS, more similar trait compositions/communities (in terms of species composition) appear closer together, thus enabling comparisons of communities. We used an individual-based species by trait matrix that included abundance per trait state in ten-day periods per period. To reduce the influence of dominant taxa and species traits the continuous variable wingspan was transformed into categorical variables with two levels (> 37 mm large and < 37 mm small), range < 29 countries small range > 30 countries large range, and colour pattern variation with three levels (0,1,2) (Clarke and Warwick 2001). The cutoffs for wingspan and range were based on the respective variable’s average value. Nonmetric multidimensional scaling (NMDS) analysis was performed on a trait state-by ten-day time period matrix, including moth abundance data (number of individuals per trait state among the 24 ten-day sampling periods) to describe the general trait composition. The NMDS was performed with the function ‘metaMDS’ in the R package vegan (Oksanen et al. 2019) using the Bray–Curtis dissimilarity measure and default settings (with three dimensions, stress = 0.057). In the NMDS, ordination communities are well represented in the dimensions if the stress is > 0.10. (Clarke and Warwick 2001). To evaluate whether the difference in trait composition between the historical and the contemporary moth community was statistically significant, we used multivariate ANOVA (function adonis2 in R package vegan). To illustrate relationships between the species composition and time period, we used the function envfit to fit the period factors onto the ordination on three axes with 999 permutations.
Results
Species richness, colonisations, and local extinctions
Analyses of the province records for Gästrikland show that the cumulative number of species increased from 522 in the historical period to 633 in 2019, corresponding to an influx of about 2.5 new species per year (Fig. 2a). A total of 34,064 individuals representing 410 species were captured in the light trap in Gävle; of these, 11,691 individuals representing 309 species were captured in the historical time period, and 22,373 individuals and 359 species in 2019. Based on information from the light trap, 152 species had either colonised (101 species, 25%) or disappeared (51 species, 12%) between the time periods, corresponding to an estimated influx of about 2.5 new species per year and a local extinction rate of about 1.16 species per year. More than half of the species (258, 63%) were captured in both periods (Fig. 2ab). Many (n = 223) species that were recorded in the province records were not captured in the light trap in any of the two time periods (Fig. 2b).
Comparisons of species richness and abundance between historical and contemporary moth communities. a Accumulated number of moths recorded from the province Gästrikland since 1975 as a line and the number of species recorded in the light trap in Gävle in the historical period and 2019. b The number of species that were present in both the historical period and in 2019 (green bar), the number of species that have been recorded in the province Gästrikland but that were not captured in the light trap site (grey bar), the number of species that went locally extinct in the trap site (red bar) and the number of species that colonized the trap site (blue bar). c Observed number of individuals caught by the light trap per night per time period. Brown/pink denotes historical period, turquoise/blue denotes 2019. The time period 13–26 August is missing because catches of moths were pooled for these weeks. d Observed species richness caught by the light trap per night across the season in historical and contemporary periods. Blue denotes historical period; orange denotes contemporary period. 13–26 August is missing because catches of moths were pooled for these weeks. e The distribution of species that disappeared or colonized the trap site across the season in historical and contemporary periods. Orange denotes colonizing species; blue denotes locally extinct species. The time period 13–26 August is missing because catches of moths were pooled for these weeks. f Bars indicate the difference in the number of individuals caught by the light trap between the contemporary and historical periods (the left Y-axis). Colonising species are in red bars, extinct species in blue and stable species in grey. The line represents total abundance in the contemporary and historical periods (right X-axis). g The relationship between abundance (log-transformed (number of individuals + 1) in the historical period and in 2019. (Color figure online)
Species richness and abundance
The results of the rarefaction analysis indicate that sampling intensity was adequate and comparable in both periods, as species saturation was met after about 100 trapping nights in both periods (Fig. S2). The rarefaction also estimated more species to be captured in the contemporary period compared to the historical period (Fig. S2). This result was confirmed when comparing nightly catches that had a statistically significantly lower species richness in all four years in the historical period compared to the contemporary period (Fig S3).
The number of species and individuals caught in the light trap per night was constantly higher across the season in 2019, and the season was 15 days longer in 2019 compared to the historical period (Fig. 2c, d). Species that went locally extinct (disappeared) typically were active in June and July, whereas new species that were first captured in 2019 were active in August, September, and October (Fig. 2e). Abundance was lower overall in the historical time period (37.8 ± 5.03, n = 309 species) than in 2019 (62.3 ± 10.4, n = 359) (Mann–Whitney–Wilcoxon test, W = 72,593, p < 0.001, n = 410 species). Two hundred twenty-four species increased in abundance, and 166 species decreased in abundance. Thirty-four species had increased with > 100 individuals, and the largest increase was found in species Orthosia gothica with 1820 individuals. Species Apamea lateritia decreases the most with −509 individuals (Fig. 2f). The most abundant species was Orthosia gothica in both periods (2495 individuals in 2019 and 675 in the historical time period). Eilema depressa was the most abundant of the colonising species found in 769 individuals in 2019. The previously most common species that had gone extinct was Apamea sordens, with 157 captured individuals in the historical time period. Species abundance in the historical time period was statistically significantly associated with the abundance in 2019 (r = 0.66, p < 0.001, Fig. 2 g).
Associations of abundance shifts with species traits
The variation among species in the direction and magnitude of abundance shifts (including new colonisations and local extinctions) between time periods was associated with two of the five species traits that we examined (Fig. 3; Table 1). The best-fitting model (lowest AIC) included two species traits (AIC = 5275, Fig. 3; Table 1), and provided a better representation of the data than the null model that only included the random terms (AIC = 5301). Results thus provide evidence for ecological filtering across species traits. Overall, species abundance increased with increasing colour pattern variation (p = 0.049) and increasing range size (Figs. 3, S4). However, the statistical significance of the association with range size (p = 0.154) fell short of the traditional critical level (Figs. 3a, S4; Table 1). Abundance shifts were not associated with diet specialisation, habitat use or wingspan.
Associationsof abundance shiftwithspeciestraits. Effect plots from the GLMM with the lowest AIC showing abundance shifts in relation to species trait a colour variation and b range size. Prediction lines derived from Linear Mixed-Effects Models with the Gaussian distribution. All models included taxonomic group as a random factor. Sample sizes are n species = 410. Statistical parameter values associated with each species trait are provided in Table 1 and figures with raw data in Fig. S4
Comparisons of community-wide multidimensional trait distributions
In the NMDS, the trait distributions were well represented both in the historical community (lowest stress solution reached at two dimensions; stress = 0.026) and in the contemporary community (lowest stress solution reached at two dimensions; stress = 0.058). The results generated by the NMDS illustrated and confirmed that the trait composition of the moth community in the contemporary period was distributed across a much smaller dimensional space than in the historical period (Fig. 4). There was no statistically significant separation in the dimensional space between the historical and contemporary moth communities (permutational multivariate ANOVA, R2 = 0.035, F1,43 = 1.01, p = 0.37). Yet, the spatial distributions of observations suggest that the overlap in the two-dimensional trait space was only ca. 35% (Fig. 4).
Two dimensional NMDS illustrating the trait composition in the historical and contemporary moth communities. The polygons show the outer limits of each period where dots are historical period and stars contemporary period. Each star and each dot represent data for a trait state-by ten-day time period. In the NMDS ordination of trait composition, communities generally occupied overlapping areas in ordination space but a significantly smaller area in the contemporary period. The stress of 0.026 for the historical period and 0.058 for the contemporary period indicated that the communities were well represented in the two reduced dimensions
The trait states that changed the most between the historical and contemporary time periods were diet specialists that increased by 239% in abundance (n = 1341 versus 4547), followed by species with a large range 156% (n = 5544 versus 14,207), high colour pattern variation 151% (n = 4540 versus 11,397), small wingspan 136% (n = 6808 versus 16,117) and habitat generalists 114% (n = 7299 versus 15,650).
Discussion
By analysing a long-term dataset spanning 50 years and including captures of over 34,064 moth individuals distributed across 410 species in central Sweden, we show that there has been a substantial net increase in species richness and total abundance. The results also uncovered considerable heterogeneity in abundance trends among different species. This variability was associated with species traits, with species characterised by high colour pattern variability and large ranges generally increasing in abundance. As a result of the shifts in species richness, species composition, and relative abundance, the phenotypic trait distribution in the contemporary moth community became more homogenous, and possibly less functional and resilient compared to the historical community (Oliver et al. 2015).
The finding of a dramatic overall increase in abundance and species richness is inconsistent with many recent studies (Franzen and Johannesson 2007; Nilsson et al. 2008; Sánchez-Bayo and Wyckhuys 2019). However, previous reports show increases in richness and abundance from studies conducted at higher latitudes (Antão et al. 2020; Keret et al. 2020) and in freshwater ecosystems (van Klink, 2020). The present study was conducted in a high latitude transitional boreonemoral zone dominated by mixed forests, relatively species-poor moth communities, and characterised by shorter and brighter nights, less light pollution, lower temperatures, low human population densities, compared with many areas in the south. This might relax the biotic and abiotic pressures on natural populations, allowing species with certain traits to increase their population growth and expand their ranges (Audusseau et al. 2021).
The colonisation rate was 2.42 and 2.52 species per year in the trap and the province, respectively, suggesting that dramatic increases occur at larger spatial scales across the whole region. This positive net shift in species richness is different from the depressing population declines and elevated extinction rates reported from lower latitudes, with high human populations and negative impacts of invasive species, land-use intensification, and habitat destruction (Merckx and Van Dyck 2019; van Langevelde et al. 2018; Wagner et al. 2021). In agreement with this interpretation, recent results based on analyses of moths in Sweden and Finland indicate that the rate of northward range expansions (Forsman et al. 2016) and increases in species richness (Antão et al. 2020) depends on latitude. There are also reports of increases in species richness being paralleled by declines in the total abundance of individuals (Antão et al. 2020). In contrast, we found that both species richness and the total abundance increased over time. This exemplifies that biodiversity responses to environmental changes vary across regions (Crossley et al. 2021).
On average, we observed a drastic change in species turnover and community composition, with 1.15 species per year disappearing from the trap site. These local extinctions probably reflect in part that forestry and agricultural practices changed between the historical and contemporary period (Franzen and Johannesson 2007; Nilsson et al. 2008; Sánchez-Bayo and Wyckhuys 2019). Our study site has faced similar environmental changes as other areas at these latitudes. Our results also seem to capture shifts in the moth’s community that generally agree with previous findings from such areas (Carvalheiro et al. 2020; Halsch et al. 2021; Leclère et al. 2020; Pöyry et al. 2016). The turnover rate that we observed (38% of the species) is alarming and calls for concern. If maintained, this dramatic turnover rate implies that a new and different community will have been established in a hundred years.
Species with boreal distributions were over-represented amongst those that declined and went extinct locally. One example is Entephria caesiata (Fig. 1b), a formerly abundant moth of boreal and montane to the subalpine coniferous forest, that could not be found in the area in 2019. Another locally extinct species was Macaria carbonaria, confined to wetlands and bogs. Species associated with flower-rich grasslands, such as Autographa bractea, also showed substantial declines. On the contrary, more southernly generalist species, such as Xestia c-nigrum and Eilema depressa were new to the area in 2019. Other generalists, such as Xestia baja and Orthosia gothica, showed a large increase in abundance in 2019 compared to the historical period. Our results suggest that while some species decline in abundance and contract their ranges, other species increase in abundance and expand their ranges, indicating a major biogeographical reshuffling of the whole fauna from a boreal to one previously associated with more southern latitudes. As the loss of diversity is irreversible, it should be one of the greatest challenges in the history of humankind (Ceballos et al. 2020). Pressures such as artificial light pollution, soil eutrophication/nitrification, urbanisation, and increasing temperatures contribute to rapid shifts in the environmental conditions on which all species depend (Carvalheiro et al. 2020; Jackson 2021; Newbold et al. 2015; van Langevelde et al. 2018).
Novel ecosystems are considered a global threat under climate change. We found that the composition and community-wide distribution of phenotypic traits tended to decrease from historical to contemporary. This conforms with reports of an ongoing global functional homogenisation (Clavel et al. 2011). Specialist species are adversely affected by past and current global changes that elevate extinction risks (Kotiaho et al. 2005; Sánchez-Bayo and Wyckhuys 2021). Generalist species have effectively replaced specialist species, bringing functional homogenisation at the community level (Franzén et al. 2020; Manlick and Newsome 2021). As moths are crucial as food for higher trophic levels, important herbivores, associated with diverse host-parasite networks and pollinators of wild plants and crops, there is potential for the functional homogenisation of moth communities demonstrated here to cascade up and down, thereby altering ecosystem functioning, goods, and services (Wang et al. 2021).
We found that differences in phenotypic traits could partly account for the heterogeneity of abundance shifts among species. Previous studies have revealed temporal variation emphasising declines in species richness, abundance and trait composition among arthropods and Lepidoptera (Coulthard et al. 2019; Habel et al. 2016, 2019, 2022; Seibold et al. 2019). In our study, we found that the shifts in species richness and abundance were generally associated with variable colour patterns and range size. Increasing abundance with increasing colour pattern variation and range size were expected, and consistent with earlier findings that variable colour pattern is associated with ecological generalisation and buffers against environmental changes (Betzholtz et al. 2017, 2019; Forsman et al. 2015, 2020; Franzén et al. 2020). This suggests that species with a variable colour pattern and wide range have become progressively more common and probably reflects an underlying association with broad niches and ecological generalisation that promotes establishment success (Forsman 2014) and buffers against current rates of environmental changes (Bartomeus et al. 2013; Mattila et al. 2011; Valtonen et al. 2017). We also found interesting seasonal patterns and the season was 15 days longer in 2019 compared to the historical period enabling species to utilise a longer season and potentially increase their abundance in the contemporary period.
Conclusions and future directions
In conclusion, our analyses of province records and light-trap data collected from a region in central Sweden provide evidence that there has been a substantial net increase in species richness and abundance of moths during the last five decades. The results also point to considerable heterogeneity in abundance trends among species that could be partially accounted for by differences in ecological and phenotypic traits. Together, these shifts translated into a community-wide trait homogenisation.
The apparent context specificity of net change estimates indicated by our findings might explain why prior attempts to synthesize insect declines have arrived at different conclusions (Crossley et al. 2020; Lister and Garcia 2018; Schowalter et al. 2021; Thomas et al. 2019; van Klink et al. 2020; Wagner et al. 2021), and calls for future systematic, comparative and meta-analytical approaches to identify the environmental and organism-related factors that contribute to the variable responses.
References
Aarvik L, Bengtsson B, Elven H, Ivinskis P, Jürivete U, Karsholt O, Savenkov N (2017) Nordic-baltic checklist of Lepidoptera. Nor J Entomol Supplement 3:1–237
Anonymous (1996) Sveriges geografi. Sveriges Nationalatlas. - Lantmäteriet
Anonymous (2020) Version 2.2; 7 July 2020. - Swedish Environmental Protection Agency, Nationella marktäckedata 2018 basskikt: Produktbeskrivning [National Land-Cover Data 2018 Basic Layer: Product Description]
Antão LH, Pöyry J, Leinonen R, Roslin T (2020) Contrasting latitudinal patterns in diversity and stability in a high-latitude species-rich moth community. Glob Ecol Biogeogr 29:896–907
Audusseau H, Ryrholm N, Stefanescu C, Tharel S, Jansson C, Champeaux L, Janz N (2021) Rewiring of interactions in a changing environment: nettle-feeding butterflies and their parasitoids. Oikos 130:624–636
Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R (2013) Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc of Natl Acad Sci 110:4656
Betzholtz P-E, Pettersson LB, Ryrholm N, Franzén M (2013) With that diet, you will go far: trait-based analysis reveals a link between rapid range expansion and a nitrogen-favoured diet. Proc Royal Soc B 280:1–6
Betzholtz PE, Franzén M, Forsman A (2017) Colour pattern variation can inform about extinction risk in moths. Anim Conserv 20:72–79
Betzholtz P-E, Forsman A, Franzén M (2019) Inter-individual variation in colour patterns in noctuid moths characterizes long-distance dispersers and agricultural pests. J Appl Entomol 143:992–999
Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Carvalheiro LG, Kunin WE, Keil P, Aguirre-Gutiérrez J, Ellis WN, Fox R, Biesmeijer JC (2013) Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol Lett 16:870–878
Carvalheiro LG, Biesmeijer JC, Franzén M, Aguirre-Gutiérrez J, Garibaldi LA, Helm A, Kunin WE (2020) Soil eutrophication shaped the composition of pollinator assemblages during the past century. Ecography 43:209–221
Ceballos G, Ehrlich PR, Raven PH (2020) Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc Natl Acad Sci 117:13596–13602
Chao A, Chazdon RL, Colwell RK, Shen TJ (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8:148–159
Clarke KR, Warwick R (2001) Change in marine communities. Approach Stat Anal Interpret 2:1–168
Clavel J, Julliard R, Devictor V (2011) Worldwide decline of specialist species: toward a global functional homogenization? -. Front Ecol Environ 9:222–228
Coleman BD, Mares MA, Willig MR, Hsieh Y-H (1982) Randomness, area, and species richness. Ecology 63:1121–1133
Coulthard E, Norrey J, Shortall C, Harris WE (2019) Ecological traits predict population changes in moths. Biol Conserv 233:213–219
Crossley MS, Meier AR, Baldwin EM, Berry LL, Crenshaw LC, Hartman GL, Moran MD (2020) No net insect abundance and diversity declines across US long term ecological research sites. Nat Ecol Evol 4:1368–1376
Crossley MS, Smith OM, Berry LL, Phillips-Cosio R, Glassberg J, Holman KM, Snyder WE (2021) Recent climate change is creating hotspots of butterfly increase and decline across North America. Glob Change Biol 27:2702–2714
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B (2014) Defaunation in the Anthropocene. Science 345:401
Douwes P (1974) Intressantare fynd av macrolepidoptera i Sverige 1973. Entomologisk Tidskrift 95:190–191
Forsman A (2014) Effects of genotypic and phenotypic variation on establishment are important for conservation, invasion, and infection biology. Proc Natl Acad Sci 111:302–307
Forsman A, Betzholtz P-E, Franzén M (2015) Variable coloration is associated with dampened population fluctuations in noctuid moths. Proc Royal Soc B 282:20142922
Forsman A, Betzholtz P-E, Franzén M (2016) Faster poleward range shifts in moths with more variable colour patterns. Sci Rep 6:36265
Forsman A, Polic D, Sunde J, Betzholtz P-E, Franzén M (2020) Variable colour patterns indicate multidimensional, intraspecific trait variation and ecological generalization in moths. Ecography 43:1–11
Fox J, Weisberg S, Price B, Adler D, Bates D, Baud-Bovy G, Bolker B (2019) car: companion to applied regression. R package version 3:0–2
Franzen M, Johannesson M (2007) Predicting extinction risk of butterflies and moths (Macrolepidoptera) from distribution patterns and species characteristics. J Insect Conserv 11:367–390
Franzén M, Schweiger O, Betzholtz P-E (2012) Species-area relationships are controlled by species traits. PLoS One 7:e37359
Franzén M, Betzholtz P-E, Pettersson LB, Forsman AJ (2020) Urban moth communities suggest that life in the city favours thermophilic multi-dimensional generalists. Proc Royal Soc B 287:20193014
Gaston KJ (1991) The magnitude of global insect species richness. Conserv Biol 5:283–296
Gilliam FS (2006) Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J Ecol 94:1176–1191
Gossner MM, Lewinsohn TM, Kahl T, Grassein F, Boch S, Prati D, Allan E (2016) Land-use intensification causes multitrophic homogenization of grassland communities. Nature 540:266–269
Gotelli N, Colwell R (2011) Estimating species richness. In: Magurran A, McGill B (eds) Frontiers in measuring biodiversity. Oxford University Press, New York, pp 39–54
Habel JC, Segerer A, Ulrich W, Torchyk O, Weisser WW, Schmitt T (2016) Butterfly community shifts over two centuries. Conserv Biol 30:754–762
Habel JC, Trusch R, Schmitt T, Ochse M, Ulrich W (2019) Long-term large-scale decline in relative abundances of butterfly and burnet moth species across south-western Germany. Sci Rep 9:1–9
Habel JC, Schmitt T, Gros P, Ulrich W (2022) Breakpoints in butterfly decline in Central Europe over the last century. Sci Total Environ 851:158315
Halsch CA, Shapiro AM, Fordyce JA, Nice CC, Thorne JH, Waetjen DP, Forister ML (2021) Insects and recent climate change. Proc Natl Acad Sci 118:e2002543117
Hellmann JJ, Fowler GW (1999) Bias, precision, and accuracy of four measures of species richness. Ecol Appl 9:824–834
Jackson ST (2021) Transformational ecology and climate change. Science 373:1085–1086
Keret NM, Mutanen MJ, Orell MI, Itämies JH, Välimäki PM (2020) Climate change-driven elevational changes among boreal nocturnal moths. Oecologia 192:1085–1098
Kotiaho J, Kaitala V, Komonen A, Päivinen J (2005) Predicting the risk of extinction from shared ecological characteristics. Proc Natl Acad Sci USA 102:1963–1967
Leclère D, Obersteiner M, Barrett M, Butchart SHM, Chaudhary A, De Palma A, Young L (2020) Bending the curve of terrestrial biodiversity needs an integrated strategy. Nature 585:551–556
Leinonen R, Söderman G, Itämies J, Rytkonen S, Rutanen I (1998) Intercalibration of different light-traps and bulbs used in moth monitoring in northern Europe. Entomologica Fennica 9:37–51
Lister BC, Garcia A (2018) Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc Natl Acad Sci 115:E10397
Lüdecke D (2018) Ggeffects: tidy data frames of marginal effects from regression models. J Open Source Softw 3:772
Macgregor CJ, Williams JH, Bell JR, Thomas CD (2019) Moth biomass increases and decreases over 50 years in Britain. Nat Ecol Evol 3:1645–1649
Manlick PJ, Newsome SD (2021) Adaptive foraging in the Anthropocene: can individual diet specialization compensate for biotic homogenization? Front Ecol Environ 19:510–518
Mattila N, Kaitala V, Komonen A, Päivinen J, Kotiaho JS (2011) Ecological correlates of distribution change and range shift in butterflies. Insect Conserv Divers 4:239–246
Merckx T, Van Dyck H (2019) Urbanization-driven homogenization is more pronounced and happens at wider spatial scales in nocturnal and mobile flying insects. Glob Ecol Biogeogr 28:1440–1455
Merckx T, Kaiser A, Van Dyck H (2018) Increased body size along urbanization gradients at both community and intraspecific level in macro-moths. Glob Change Biol 24:3837–3848
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko I, Senior RA, Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50
Nilsson SG, Franzén M, Jonsson E (2008) Long-term land-use changes and extinction of specialised butterflies. Insect Conserv Divers 1:197–207
Nordström F (1943) Catalogus insectorum Sueciae. III. Macrolepidoptera. Opusc Ent 8:59–120
Nordström F (1952) Hur noggrant känna vi utbredningen av Sveriges Macrolepidoptera? Opuscula Entomologica 17:209–211
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) vegan: Community vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan
Oliver TH, Heard MS, Isaac NJ, Roy DB, Procter D, Eigenbrod F, Petchey OL (2015) Biodiversity and resilience of ecosystem functions. Trends Ecol Evol 30:673–684
Palacio FX (2020) Urban exploiters have broader dietary niches than urban avoiders. Ibis 162:42–49
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Warren M (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–584
Pimm SL, Lee HJ, Diamond J (1988) On the risk of extinction. Am Nat 132:757–785
Pöyry J, Carvalheiro LG, Heikkinen RK, Kühn I, Kuussaari M, Schweiger O, Franzén M (2016) The effects of soil eutrophication propagate to higher trophic levels. Glob Ecol Biogeogr 26:18–30
R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. R version 4.0.3. https://www.R-project.org
Reese GC, Wilson KR, Flather CH (2014) Performance of species richness estimators across assemblage types and survey parameters. Glob Ecol Biogeogr 23:585–594
Richardson LE, Graham NAJ, Pratchett MS, Eurich JG, Hoey AS (2018) Mass coral bleaching causes biotic homogenization of reef fish assemblages. Glob Change Biol 24:3117–3129
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27
Sánchez-Bayo F, Wyckhuys KA (2021) Further evidence for a global decline of the entomofauna. Austral Entomol 60:9–26
Schowalter TD, Pandey M, Presley SJ, Willig MR, Zimmerman JK (2021) Arthropods are not declining but are responsive to disturbance in the Luquillo Experimental Forest, Puerto Rico. Proc Natl Acad Sci 118:e2002556117
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarlı D, Ammer C, Bauhus J, Fischer M, Habel JC (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674
Soroye P, Newbold T, Kerr J (2020) Climate change contributes to widespread declines among bumble bees across continents. Science 367:685–688
Thomas CD, Jones TH, Hartley SE (2019) “Insectageddon”: a call for more robust data and rigorous analyses. Glob Change Biol 25:1891–1892
Valtonen A, Hirka A, Szőcs L, Ayres MP, Roininen H, Csóka G (2017) Long-term species loss and homogenization of moth communities in Central Europe. J Anim Ecol 86:730–738
van Klink R, Bowler DE, Gongalsky KB, Swengel AB, Gentile A, Chase JM (2020) Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368:417–420
van Langevelde F, Braamburg-Annegarn M, Huigens ME, Groendijk R, Poitevin O, van Deijk JR, WallisDeVries MF (2018) Declines in moth populations stress the need for conserving dark nights. Glob Chang Biol 24:925–932
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the Anthropocene: death by a thousand cuts. Proc Natl Acad Sci 118:e2023989118
Wang S, Loreau M, de Mazancourt C, Isbell F, Beierkuhnlein C, Connolly J, Hector A (2021) Biotic homogenization destabilizes ecosystem functioning by decreasing spatial asynchrony. Ecology 102:e03332
Acknowledgements
We thank Eliza Grames and one anonymous referee for comments on a previous version of this manuscript, and Lena Carlsson for entering trap records to excel.
Funding
Open access funding provided by Linnaeus University. The study was funded by the Swedish National Research Programme on Climate through the Swedish Research Council for Sustainable Development, Formas (Grant to M.F. and A.F. Dnr. 2018-02846 and 2021-02142), Carl Tryggers Stiftelse för Vetenskaplig Forskning, and Linnaeus University.
Author information
Authors and Affiliations
Contributions
AF and MF conceived the study. GS did the fieldwork and collected the data. YF analyzed the data under the supervision of AF and MF. AF, MF, and YF interpreted the data/results and wrote the first draft. All authors commented on the manuscript and approved the final version before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franzén, M., Francioli, Y., Sjöberg, G. et al. Positive shifts in species richness and abundance of moths over five decades coincide with community-wide phenotypic trait homogenisation. J Insect Conserv 27, 323–333 (2023). https://doi.org/10.1007/s10841-023-00458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00458-y