Abstract
Introduction
Insects are reported to be in decline around the globe, but long-term datasets are rare. The causes of these trends are elusive, with changes in land use and climate among the top candidates. Yet if species traits can predict rates of population change, this can help identify underlying mechanisms. If climate change is important, for example, high-latitude species may decline as temperate species expand. Land use changes, however, may impact species that rely on certain habitats.
Aims and methods
We present 30 years of moth captures (comprising 97,032 individuals of 808 species) from a site in southeast Norway to test for population trends that are correlated with species traits. We use time series analyses and joint species distribution models combined with local climate and habitat data.
Results and discussion
Species richness declined by 8.2% per decade and total abundance appeared to decline as well (−9.4%, p = 0.14) but inter-annual variability was high. One-fifth of species declined, although 6% increased. Winter and summer weather were correlated with annual rates of abundance change for many species. Opposite to general expectation, many species responded negatively to higher summer and winter temperatures. Surprisingly, species’ northern range limits and the habitat in which their food plants grew were not strong predictors of their time trends or their responses to climatic variation. Complex and indirect effects of both land use and climate change may play a role in these declines.
Implications for insect conservation
Our results provide additional evidence for long-term declines in insect abundance. The multifaceted causes of population changes may limit the ability of species traits to reveal which species are most at risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects are reported to be in decline around the globe, based on evidence spanning many different ecosystems and at both local and regional scales (Sánchez-Bayo and Wyckhuys 2019, Didham et al. 2020, van Klink et al. 2020, Wagner 2020). These declines may be due to a host of factors, including land use changes (especially agricultural intensification), pesticides and pollution (including light pollution), introduced species, and climate change (Habel et al. 2019a, Seibold et al. 2019, Raven and Wagner 2021, Wagner et al. 2021).
Detecting and measuring declines, however, requires long-term datasets, and these are rare (Didham et al. 2020, Montgomery et al. 2020). Continuous long-term datasets, rather than comparisons between several time points (Seibold et al. 2019), are especially important for taxa (like many insects) that exhibit population cycles and high inter-annual variability (Welti et al. 2020). Unfortunately, there are few such datasets from Fennoscandia, even though high-latitude systems are experiencing some of the strongest effects of climate change (Hunter et al. 2014, Loboda et al. 2017).
Insects are a hyper-diverse class, and no single sampling scheme can adequately monitor all groups (Montgomery et al. 2020). Lepidopterans, however, may be good early indicators of broader changes to insect communities (Sánchez-Bayo and Wyckhuys 2019, Montgomery et al. 2020), in part because they may be particularly susceptible to environmental change (Hunter et al. 2014). They are also important for pollination, a valuable ecosystem service (Macgregor et al. 2015). Moths, which are much more diverse than butterflies, serve as an important food source for birds, bats and other insectivorous taxa (Sánchez-Bayo and Wyckhuys 2019).
Lepidopterans may also be among those insect groups that are declining most rapidly (Sánchez-Bayo and Wyckhuys 2019). For example, moths and butterflies in the Netherlands (Van Dyck et al. 2009, Groenendijk and Ellis 2011, Langevelde et al. 2018, Hallmann et al. 2020) and the United Kingdom (Conrad et al. 2004, Roy et al. 2015, Dennis et al. 2019) appear to have experienced considerable declines in both abundance and species richness in the last 30 years. Historic baselines are inevitably relative, meaning that longer time series are more informative than shorter ones (Macgregor et al. 2019b, 2021) and critical analyses and interpretation are required (Thomas et al. 2019). Farther north, there is some evidence from Finland that total moth abundance has declined over the last 20 years, although species richness appears to have increased due to range expansions of southern species (Antão et al. 2020). Swedish butterflies inhabiting meadows and grazed open forests also appear to have declined in abundance during this same period (Franzén and Johannesson 2007, Nilsson et al. 2013).
Even as these trends in richness and abundance have been documented, their causes remain elusive; correlations with proposed causal factors provide only weak evidence. However, if trends of the individual species that make up these overall abundance patterns can be predicted using their traits (Mattila et al. 2006, Habel et al. 2019a), this can provide important clues to the underlying processes. Traits can help link species responses to land use changes (Fox et al. 2014, Coulthard et al. 2019), and can also help predict responses to climatic variation in the short term (Roy et al. 2015), reducing the noise in inter-annual population variability and providing clues to the role of climate in long-term trends in abundance.
Range limits are a trait that can be used to better understand a species’ response to climate change (Kirkpatrick and Barton 1997, Sexton et al. 2009, Fox et al. 2014). In moths, for example, species with more northerly ranges (Itämies et al. 2011) are more likely to be declining in some regions. Many of the most common species in northern Finland, however, appear to have been stable or even to have increased over the last several decades (Hunter et al. 2014). If climate change is causing long-term poleward shifts in moth species’ ranges, as has been demonstrated in some other insects (Parmesan et al. 1999, Pöyry et al. 2009, Boggs 2016), then many species in the Northern Hemisphere may be becoming more abundant near their northern range limits, or declining at their southern range edges (Warren et al. 2001). Range limits may also shift as a function of changing abundance of a species (Mair et al. 2014, Macgregor et al. 2019a).
If, on the other hand, land use change is an important contributor to population changes, these changes might be predicted by the habitat requirements of a species’ preferred food plant (Habel et al. 2019b). Moth species with higher host plant specificity are more likely to be declining than other species in Finland (Mattila et al. 2006) and Sweden (Franzén and Johannesson 2007), and biotic homogenization appears to be occurring in Hungarian moths as species that require grasslands or specialized diets are disappearing (Valtonen et al. 2017). Land use and climate change are not independent, and they have been shown to interact in affecting species range limits and shifts (Mair et al. 2014, Platts et al. 2019).
Here we examine a 30-year moth capture dataset, in which 808 species were collected using consistent methods in southeast Norway. We use time series analyses and Bayesian community modeling to ask: (a) How do moth community richness, diversity, and abundance vary through time? (b) Do rates of abundance change differ among species with different range extents or food plant habitats? (c) On an annual basis, are species’ range limits good predictors of their responses to climatic variation?
Methods
Study area
Moths were trapped each summer from 1984 to 2013 at a site (59.7477° N, 10.5925° E, 70 m) in Nesodden municipality, southeast Norway (Kobro 1991) (Fig. 1). The trap site was located on a peninsula in the Oslo Fjord, 20 km south of Oslo (Fig. 1). It was at the edge of a garden, shaded from most moonlight, and surrounded by deciduous, mixed, and coniferous forest. The locations of trees and buildings in the immediate area did not change through the years. The landscape within five kilometers of the trap site is largely forested (70%), and much of the remaining land (22%) was farmland and open areas in 1984 based on our comparison of Landsat images using the rpart R-package (Therneau and Atkinson 2019) (see Supplementary Methods). Forest cover remained relatively constant throughout the trapping period, but by 2014 half of the fields and farmland had been developed, largely for housing. These changes are similar to those that have occurred within a larger 25 km radius (Supplementary Table S1; Supplementary Fig. S1). Within the forests of surrounding Akershus county, total wood volume and tree species composition were similar in 1983 and 2013, although there was a slight shift towards more mature forests (Statistics Norway; www.ssb.no).
Sampling methods
A funnel light trap (160 W/235 V mixed light bulb) was hung 1 m above the ground in the same location through the night for the first three nights of each week from early June to mid-October, 1984–2013 as described by Kobro (1991).On nights with rain, wind, or otherwise poor trapping conditions, that night was dropped and the trap was deployed an additional night. Moths were identified using genitalia and external characters by SK. Taxonomy follows the gbif taxonomic backbone (GBIF Secretariat 2021). To summarize the abundance of each species for a given year, we filtered the dataset to include only nights within the range of dates that were sampled every year (02 July–16 October). This left an average of 42.7 sampling nights per year (range 36–46). We then divided total counts of each species per year by the number of sampling nights in that year to get mean number of individuals per night, and multiplied this value by the mean number of sampling nights (42.7), rounded to the nearest integer, to get comparable estimates of abundance among years. The filtered dataset included 808 species after excluding those species captured only outside of the core summer trapping season (n = 74).
Species traits
To determine if species’ trends and responses to weather were correlated with their geographic ranges, we assigned 336 species (those captured in 10 or more years) to one of three groups based on their ranges in Norway, using information from Bengtsson et al. (2008), Bengtsson and Johansson (2011), Svensson (2006), and Artsdatabanken (www.artsdatabanken.no). These included: ‘southern’ species (n = 90), which occur only in the south of Norway (below 60.5° N), ‘central’ species (n = 113), which occur as far north as central Norway (64° N), and ‘widespread’ species (n = 133) which occur the entire length of the country (Fig. 3).
To test for relationships between species trends and land use changes, a list of preferred food plants was also obtained for each species from Goater et al. (1986), Palm (1989), Svensson (2006), Bengtsson et al. (2008), Aarvik et al. (2009), Bengtsson and Johansson (2011), Sterling and Parsons (2012), and the authors’ observations. Using our knowledge of Norwegian plants, we then classified these groups of host plants as occurring primarily in: (a) forests (n = 162), (b) gardens (n = 24), (c) meadows (including agricultural field edges; n = 67), (d) meadows and forests (n = 51), or (e) unknown/other (n = 32).
Testing for trends
We summed the total numbers of species in the dataset by year (using the full dataset, with all 808 species from the core sampling period included). For total individuals, we used the number of individuals corrected for effort as described above. We also estimated true species richness in each year using the iChao1 metric in the SpadeR R-package (Chao et al. 2016) in R (R Core Team 2021). This metric uses the number of singletons and doubletons in a dataset to estimate the number of present but unobserved species. Finally, we calculated the Shannon diversity index for each year using the vegan R-package (Oksanen et al. 2017).
For each of these community metrics we tested for evidence of a trend through time using a modified Mann-Kendall test in the modifiedmk R package (Patakamuri and O’Brien 2020). This test is robust for highly variable non-normal count data and estimates the slope of the trend line, if significant (p < 0.05). The test requires that data are not highly autocorrelated, and we found that serial autocorrelation in our richness and diversity data was significant only at a time interval of one year (p < 0.05), making this an appropriate test.
For plotting purposes the intercept of the trend line (the estimate for year zero, 1984), which isn’t provided by the Mann-Kendall test, was calculated such that the line would pass through the median value of the response variable in year 15 of 30. We expressed rates of increase or decline as the percent change across a 10-year period relative to the intercept (Sánchez-Bayo and Wyckhuys 2019). Similarly, we also tested for a trend in each species that was detected in ten or more years (n = 336), because detecting a significant trend is difficult in species that are present at fewer time steps. Species trends were expressed as change per decade as a % of the median annual count value for that species, because many species were rare or absent early or late in the study and so a rate based on intercept values could be misleading. In cases where the median count was less than three (due to the presence of many zeros), we used the median value when present to avoid inflating rates of change. Moving averages for plotting were calculated using the ‘sma’ function in the smooth R package (Svetunkov 2020), which uses BICc to determine the number of years to include in the moving average.
Climate covariates
We summarized climate covariates for the area surrounding the trap site for each summer (June–August) and winter (December–March) of the study period using estimates from the ERA5 climate reanalysis (Copernicus Climate Change Service [C3S] 2017). Values were taken from the grid cell (0.5° latitude x 0.5° longitude) surrounding the trap site. These included mean summer air temperature (at 2 m), cumulative summer precipitation, mean winter soil temperature (at 1 cm depth), and mean winter snow depth. We chose mean winter soil temperature, rather than air temperature, to better represent the temperature that overwintering eggs, larvae and pupae were subjected to.
Responses to covariates
To test the hypothesis that population growth rates respond to inter-annual variation in weather, with southern species benefiting from more benign weather, we fit Bayesian hierarchical joint species distribution models (JSDMs) using the Hmsc R-package (Ovaskainen et al. 2017a, 2017b). We modeled population rate of change (capture rates relative to previous year) as a function of summer and winter temperature and precipitation, based on estimates from the ERA5 climate reanalysis (C3S 2017). Determining a population rate of change for a given year requires capture records from that year as well as from the previous year; we included all species for which rates of change could be calculated in ten or more years (n = 184). To calculate rate of population change r, we used log-transformed abundance data for each count C of species s in year t:
For species that were not captured either in a given year or in the preceding year, the rate of population change response variable in that year was treated as missing (NA). These JSDMs jointly estimate the response of each species to the environmental covariates, incorporating trait (species range) and phylogenetic information as a hierarchical level to estimate whether functionally or phylogenetically related species respond to the environment in similar ways. We included a phylogeny based on the species-level insect tree from Chesters (2017) to test for phylogenetic signal in the responses. Missing species were added to the correct genus (when present) or family using the ape R package (Paradis and Schliep 2019). Year was included in models as a temporally structured random effect.
We fit a series of six community models (Table 1) with Gaussian error distributions and an identity link function. Models differed in their combinations of environmental covariates (including covariates from the previous year, to test for lag effects). Models were fitted using five MCMC chains with 6800 iterations each. The first 5000 iterations of each chain were discarded as burn-in, and the remaining iterations were thinned by 6 to produce 300 samples per chain (1500 in total). Model fitting was conducted with high performance computational resources provided by Louisiana State University (http://www.hpc.lsu.edu), and models were ranked using the widely-applicable information criterion (WAIC) from Watanabe (2010).
Map of the long-term moth trapping site in southeast Norway. Moths were captured several nights per week from early June to mid-October, 1984–2013. The central black point represents the trap site, which was situated on a garden edge surrounded by mixed forest. Inset shows location in northern Europe
Results
We recorded 808 moth species and 97,032 individuals in 30 years of sampling during the core summer trapping period. These species came from 43 families in 14 superfamilies (Supplementary Table S2). and were all used to calculate the community metrics. An additional 74 species (11,308 individuals) were detected only outside the core summer trapping period and were excluded from all analyses. Annual observed species richness ranged from 217 to 429 (mean = 284.9, sd = 48.5). Of 808 total species, 336 were captured in ten or more years and were used to calculate species-specific metrics.
Trends in community metrics
Observed and estimated species richness, as well as diversity, decreased through the study period (Fig. 2), based on Mann-Kendall tests (alpha = 0.05). Observed species richness declined by 8.2% per decade (p < 0.01), and abundance of individuals appeared to decline as well (−9.4% per decade; p = 0.14); most seasons in the later years of the study included 50–100 fewer species than most seasons earlier in the study.
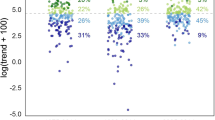
Moth species were assigned to three groups (southern, central, and widespread species) based on their ranges in Norway (Fig. 3), because of the expectation that more southerly species may respond more positively to climate change (Warren et al. 2001). When these groups with different ranges were considered separately, species richness declined for each group (range: −5.7% to −7.3% per decade; Supplementary Fig. S2). The number of individuals captured declined (−17.0% per decade) for widespread species (driving the decreasing trend in overall abundance), whereas we did not detect overall abundance changes in the other groups (Supplementary Fig. S3).
Trends in total observed richness, estimated species richness (iChao1), total individuals, and Shannon diversity from a 30-year moth capture dataset from southeastern Norway (10-year rates of decline: −8.2%, −8.1%, −9.4%, and −5.0%, respectively). Plots show raw values (blue), moving average (black), and trend (red) lines. Slopes were estimated using a modified Mann–Kendall test and plotted such that the line passes through the median value in year fifteen of the study. Counts of total individuals were standardized by number of trapping nights. Significance of slopes is indicated at the 0.05 (*), 0.01 (**), and 0.001 (***) levels. The number of time points included in each moving average (10, 5, 15, and 11, respectively) was selected using BIC. (Color figure online)
Three moth species representing those with northern range limits in northern (top; ‘widespread’ species), central (middle), and southern (bottom) Norway. Plots (center column) show raw values (blue), moving average (black), and trend lines (red) based on captures from a site in southeastern Norway. Maps of detections (right) are from Artsdatabanken (artsdatabanken.no). Slopes were estimated using a modified Mann–Kendall test and plotted such that the line passed through the median value in year fifteen of the study. Significance of the slope is indicated at the 0.05 (*) level. The number of time points included in each moving average (12, 2, and 12, respectively) was selected using BIC. (Color figure online)
Trends in individual species
We also tested for time trends in the 336 species that were detected in ten or more years (Supplementary Table S3). Of these, 18% (n = 59) show a negative trend (Mann–Kendall test; alpha = 0.05) and 6% a positive trend (n = 19; Table 2). The 10-year rate of change for the declining species varied from − 14% to − 111% (median = − 50%) relative to the median annual count, and for the increasing species the rate of change varied from 7% to 155% (median = 44%). There was some evidence that mean rates of population change differed among species with different ranges (df = 2, F = 2.46, p = 0.087; Fig. S4). Southern species were somewhat less likely to be decreasing and widespread species somewhat more likely to be decreasing than expected by chance (Chi-squared test, df = 4, X2 = 8.559, p value = 0.073; Table 2), based on standardized residuals.
We also classified species based on the habitat (forest, garden, meadow, or forest/meadow) of their preferred food plants, to test the hypothesis that land use change was contributing to population trends (Habel et al. 2019b). Trends in these species did not differ by habitat group (df = 4, F = 1.58, p = 0.178), nor did the proportion of species that were increasing or declining (Table 3; Chi-squared test, df = 8, X2 = 6.415, p value = 0.601). Trend estimates for each species, along with range and habitat designations and total numbers of years and individuals detected, are shown in Supplementary Table S3.
Responses to environmental covariates
Model convergence and mixing were good, based on effective sample sizes and potential scale reduction factors. The two best-performing models for predicting species’ rates of change included climatic covariates from winter (soil temperature and snow depth; Table 1) and summer (temperature and precipitation) of year t. Models including all covariates, or lag covariates from the previous year (t-1) were ranked lower. Many species responded negatively to increased winter soil temperature, summer temperature, and summer precipitation (Table 4). Responses to winter snow depth were mixed and less common. Winter soil temperature and summer precipitation explained the most variation in their respective models (Supplementary Fig. S5). Responses to covariates did not differ among species with different ranges (95% CIs). Instead, responses were strongly correlated with phylogeny (95% CI: 0.93–0.99, where 1.0 indicates the strongest possible phylogenetic effect). Models explained a relatively small proportion of total differences in moth abundance between years, as indicated by the mean R2 values (Table 1).
Discussion
We analyzed a 30-year moth capture dataset from southeastern Norway and found that the richness and diversity of the community have declined considerably. Abundance of species with the most northerly range limits declined as well. Nearly one-fifth of species declined, whereas only 6% increased. Inter-annual variability in community metrics and individual species was high, even when trends were strong. Southern species appear less likely to be in decline, and widespread species more likely, although statistical support for this result was weak. This is compatible with the hypothesis that Scandinavia, which is becoming warmer and wetter (Ljungqvist et al. 2019), is becoming more hospitable to southerly species (and possibly less hospitable to more northerly species). However, opposite to a general expectation that warmer seasons could have an enriching effect on local richness and abundance of poikilothermal species like moths, rates of population change for many species in our study were negatively correlated with summer temperature and winter soil temperature. Many species also responded negatively to wetter summers. Rates of decline did not appear to differ among species whose preferred food plants grew in different types of habitat, despite the conversion of many meadows and farmlands in the surrounding area to residential or commercial property over the sampling period.
Our declines in moth richness and abundance mirror many of those found around the globe, where insect biomass and species richness appear to be falling in many ecosystems (Sánchez-Bayo and Wyckhuys 2019, Didham et al. 2020, Wagner 2020). Some communities, however, don’t follow this pattern, highlighting that biodiversity loss is a complex phenomenon (Antão et al. 2020). Moths in subarctic Finland, for example, are as a whole stable or increasing (Hunter et al. 2014, Antão et al. 2020), and biomass of British moths may be stable (Macgregor et al. 2019b, 2021) although over one third of species are declining (Fox et al. 2014). Understanding the processes that cause declines in some groups but not in others remains a challenge. Traits can be helpful in some cases (Habel et al. 2019a), but (as in our study) they often do not explain much variation in species responses (Mattila et al. 2006). This could mean that stochastic processes play a role, but given our relative ignorance about the life histories, diets, and indirect effects of climate on many species it seems likely that additional study could reveal important traits. High phylogenetic signal in species’ responses to climate covariates in our JSDMs implies that unmeasured but phylogenetically structured traits do play a role (Abrego et al. 2017).
Two potential causes of the declines we report—climate and land use changes—have occurred simultaneously and are difficult to unravel (Mair et al. 2014, Platts et al. 2019, Cardoso et al. 2020, Samways et al. 2020). Models based on annual variation in weather had some predictive value in our system, but rates of decline varied only slightly among species with different range limits (and, presumably, different climatic optima), although southern species appear less likely to be declining.
Direct effects of climate change may not be driving these community changes in straightforward ways, but climate may be important for other reasons. Changes in phenology can cause mismatches between lepidoptera and their food sources, for example, and more frequent severe weather events (including droughts) could increase strain on populations (Van Dyck et al. 2015, Boggs 2016). Climate change may cause ranges to contract for species not successful at climate tracking, as has been demonstrated for several other insect groups, including bumblebees (Soroye et al. 2020). A long-term study of Hungarian macro-moths also found that southern species were no more likely to appear in new sites than were northern species (Valtonen et al. 2017).
Forest cover has remained constant in southeastern Norway while much farm and field habitat has been lost to housing and other development, but these land use changes were poor predictors of which species were in decline. This contrasts with studies of moths in Germany, where grassland-dependent species appear to have some of the highest rates of decline (Habel et al. 2019b, c). Land use change might be less influential for moths in Fennoscandia or southeast Norway specifically, or our limited knowledge of host plant specificity for many species may reduce our ability to detect such effects. For instance, moth host plants related to field edges might still be present along roadsides in developed areas. Use of pesticides and herbicides in Norway appears to have remained relatively stable since the mid 1990’s (Stenrød 2015). That many sub-Arctic moth species in Finland appear to be stable, despite the speed at which high-latitude climates are warming, may imply that these far northern habitats that have been subject to less intensive land use can act as a buffer to stabilize populations (Hunter et al. 2014, Zellweger et al. 2020).
Regardless of the causes, the negative trends in moth populations that we report can impact entire ecosystems; for example, moths are important for pollination (Macgregor et al. 2015), and as food for insectivorous birds, many of which are also in sharp decline across Europe (Bowler et al. 2019), and for bats. Population trends for moths could also serve as an early-warning sign for other insects (Hunter et al. 2014).
One limitation of our study is that our capture records are from a single site (Hallmann et al. 2017), which limits our ability to generalize our results; rather, they should be used to form hypotheses for larger-scale monitoring projects. However, our time series is unbroken, consistent methods were used throughout, and the immediate surroundings of the trap site were unchanged. Records from long temporal and large spatial scales are rare, yet they are vital as we try to characterize insect population trends, understand their causes, and prevent precipitous declines (Montgomery et al. 2020, Samways et al. 2020). Increased long-term insect monitoring is needed. Funders should make provisions for these long-term studies that have traditionally been difficult to sustain, and study designs should allow the roles of alternative causal mechanisms to be disentangled.
Data availability
Data are available on Zenodo: https://doi.org/10.5281/zenodo.5553031
References
Aarvik L, Hansen LO, Kononenko VS (2009) Norges summerflugler: håndbok over Norges dagsommerfugler og nattsvermere. Norks entomologiks forening. Naturhistorisk museum, Oslo
Abrego N, Norberg A, Ovaskainen O (2017) Measuring and predicting the influence of traits on the assembly processes of wood-inhabiting fungi. J Ecol 105:1070–1081
Antão LH, Pöyry J, Leinonen R, Roslin T (2020) Contrasting latitudinal patterns in diversity and stability in a high-latitude species‐rich moth community. Glob Ecol Biogeogr 29:896–907
Bengtsson B, Johansson R, Palmqvist G (2008) Nationalnyckeln till Sveriges flora och fauna (Encyclopedia of the Swedish Flora and Fauna). Fjärilar: Käkmalar–säckspinnare. Lepidoptera: Micropterigidae-Psychidae. DE 1–13. ArtDatabanken, SLU, Uppsala
Bengtsson B, Johansson R (2011) Nationalnyckeln till Sveriges flora och fauna (Encyclopedia of the Swedish Flora and Fauna). Fjärilar: Bronsmalar-rullvingemalar. Lepidoptera: Roeslerstammiidae-Lyonetiidae. SLU, Uppsala DE 14-25. ArtDatabanken
Boggs CL (2016) The fingerprints of global climate change on insect populations. Curr Opin Insect Sci 17:69–73
Bowler DE, Heldbjerg H, Fox AD, Jong M, Böhning-Gaese K (2019) Long‐term declines of European insectivorous bird populations and potential causes. Conserv Biol 33:1120–1130
Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kwak ML, Mammola S, Ari Noriega J, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Settele J, Simaika JP, Stork NE, Suhling F, Vorster C, Samways MJ (2020) Scientists’ warning to humanity on insect extinctions. Biol Conserv 242:108426
Chao A, Ma KH, Hsieh TC, Chiu CH (2016) SpadeR: species-richness prediction and diversity estimation with R. R package version 0.1.1. https://CRAN.R-project.org/package=SpadeR
Chesters D (2017) Construction of a species-level tree of life for the insects and utility in taxonomic profiling. Syst Biol 66:426–439
Conrad KF, Woiwod IP, Parsons M, Fox R, Warren MS (2004) Long-term population trends in widespread British moths. J Insect Conserv 8:119–136
Copernicus Climate Change Service (C3S) (2017) ERA5: fifth generation of ECMWF atmospheric reanalyses of the global climate. Copernicus climate change service climate data store (CDS)
Coulthard E, Norrey J, Shortall C, Harris WE (2019) Ecological traits predict population changes in moths. Biol Conserv 233:213–219
Dennis EB, Brereton TM, Morgan BJT, Fox R, Shortall CR, Prescott T, Foster S (2019) Trends and indicators for quantifying moth abundance and occupancy in Scotland. J Insect Conserv 23:369–380
Didham RK, Basset Y, Collins CM, Leather SR, Littlewood NA, Menz MHM, Müller J, Packer L, Saunders ME, Schönrogge K (2020) Interpreting insect declines: seven challenges and a way forward. Insect Conserv Divers 13:103–114
Fox R, Oliver TH, Harrower C, Parsons MS, Thomas CD, Roy DB (2014) Long-term changes to the frequency of occurrence of British moths are consistent with opposing and synergistic effects of climate and land-use changes. J Appl Ecol 51:949–957
Franzén M, Johannesson M (2007) Predicting extinction risk of butterflies and moths (Macrolepidoptera) from distribution patterns and species characteristics. J Insect Conserv 11:367–390
GBIF Secretariat (2021) GBIF backbone taxonomy. Checklist dataset. https://doi.org/10.15468/39omei. Accessed 13 Sept 2021
Goater B, Senior G, Dyke R (1986) British pyralid moths: a guide to their identification. Harley Books, Colshester
Groenendijk D, Ellis WN (2011) The state of the Dutch larger moth fauna. J Insect Conserv 15:95–101
Habel JC, Samways MJ, Schmitt T (2019a) Mitigating the precipitous decline of terrestrial European insects: requirements for a new strategy. Biodivers Conserv 28:1343–1360
Habel JC, Segerer AH, Ulrich W, Schmitt T (2019b) Succession matters: community shifts in moths over three decades increases multifunctionality in intermediate successional stages. Sci Rep. https://doi.org/10.1038/s41598-019-41571-w
Habel JC, Trusch R, Schmitt T, Ochse M, Ulrich W (2019c) Long-term large-scale decline in relative abundances of butterfly and burnet moth species across south-western Germany. Sci Rep. https://doi.org/10.1038/s41598-019-51424-1
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, De Kroon H (2017) More than 75 % decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809
Hallmann CA, Zeegers T, Klink R, Vermeulen R, Wielink P, Spijkers H, Deijk J, Steenis W, Jongejans E (2020) Declining abundance of beetles, moths and caddisflies in the Netherlands. Insect Conserv Divers 13:127–139
Hunter MD, Kozlov MV, Itämies J, Pulliainen E, Bäck J, Kyrö E-M, Niemelä P (2014) Current temporal trends in moth abundance are counter to predicted effects of climate change in an assemblage of subarctic forest moths. Glob Change Biol 20:1723–1737
Itämies JH, Leinonen R, Meyer-Rochow VB (2011) Climate change and shifts in the distribution of moth species in Finland, with a focus on the province of Kainuu. In: Blanco JA, Kheradmand H (eds) Climate change–geophysical foundations and ecological effects. InTech, Rijeka, pp 273–296
Kirkpatrick M, Barton NH (1997) Evolution of a species’ range. Am Nat 150:1–23
Kobro S (1991) Annual variation in abundance of phototactic Lepidoptera as indicated by light-trap catches. Fauna norvegica Ser B 38:1–4
Langevelde F, Braamburg-Annegarn M, Huigens ME, Groendijk R, Poitevin O, Deijk JR, Ellis WN, Grunsven RHA, Vos R, Vos RA, Franzén M, Wallisdevries MF (2018) Declines in moth populations stress the need for conserving dark nights. Glob Change Biol 24:925–932
Ljungqvist FC, Seim A, Krusic PJ, González-Rouco JF, Werner JP, Cook ER, Zorita E, Luterbacher J, Xoplaki E, Destouni G, García-Bustamante E, Aguilar CAM, Seftigen K, Wang J, Gagen MH, Esper J, Solomina O, Fleitmann D, Büntgen U (2019) European warm-season temperature and hydroclimate since 850 CE. Environ Res Lett 14:084015
Loboda S, Savage J, Buddle CM, Schmidt NM, Høye TT (2017) Declining diversity and abundance of high Arctic fly assemblages over two decades of rapid climate warming. Ecography. https://doi.org/10.1111/ecog.02747
Macgregor CJ, Pocock MJO, Fox R, Evans DM (2015) Pollination by nocturnal L epidoptera, and the effects of light pollution: a review. Ecol Entomol 40:187–198
Macgregor CJ, Thomas CD, Roy DB, Beaumont MA, Bell JR, Brereton T, Bridle JR, Dytham C, Fox R, Gotthard K, Hoffmann AA, Martin G, Middlebrook I, Nylin S, Platts PJ, Rasteiro R, Saccheri IJ, Villoutreix R, Wheat CW, Hill JK (2019a) Climate-induced phenology shifts linked to range expansions in species with multiple reproductive cycles per year. Nat Commun. https://doi.org/10.1038/s41467-019-12479-w
Macgregor CJ, Williams JH, Bell JR, Thomas CD (2019b) Moth biomass increases and decreases over 50 years in Britain. Nat Ecol Evol 3:1645–1649
Macgregor CJ, Williams JH, Bell JR, Thomas CD (2021) Author correction: moth biomass has fluctuated over 50 years in Britain but lacks a clear trend. Nat Ecol Evol 5:865–883
Mair L, Hill JK, Fox R, Botham M, Brereton T, Thomas CD (2014) Abundance changes and habitat availability drive species’ responses to climate change. Nat Clim Change 4:127–131
Mattila N, Kaitala V, Komonen A, Kotiaho JS, Päivinen J (2006) Ecological determinants of distribution decline and risk of extinction in moths. Conserv Biol 20:1161–1168
Montgomery GA, Dunn RR, Fox R, Jongejans E, Leather SR, Saunders ME, Shortall CR, Tingley MW, Wagner DL (2020) Is the insect apocalypse upon us? How to find out. Biol Conserv 241:108327
Nilsson SG, Franzén M, Pettersson L (2013) Land-use changes, farm management and the decline of butterflies associated with semi-natural grasslands in Southern Sweden. Nat Conserv 6:31
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin P, O’Hara R, Simpson G, Solymos P, Stevens M, Wagner H (2017) Vegan: community ecology package. R package. https://CRAN.R-project.org/package=vegan
Ovaskainen O, Tikhonov G, Dunson D, Grøtan V, Engen S, Sæther B-E, Abrego N (2017a) How are species interactions structured in species-rich communities? A new method for analysing time-series data. Proc R Soc B 284:20170768
Ovaskainen O, Tikhonov G, Norberg A, Guillaume Blanchet F, Duan L, Dunson D, Roslin T, Abrego N (2017b) How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol Lett 20:561–576
Palm E (1989) Nordeuropas prydvinger (Lepidoptera: Oecophoridae): med saerligt henblik på den danske fauna. Fauna Bøger, København
Paradis E, Schliep K (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528
Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Huntley B, Kaila L, Kullberg J, Tammaru T, Tennent WJ, Thomas JA, Warren M (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583
Patakamuri SK, O’Brien N (2020) Modifiedmk: modified versions of Mann Kendall and Spearman’s Rho trend tests. R package version 1.5.0. https://CRAN.R-project.org/package=modifiedmk
Platts PJ, Mason SC, Palmer G, Hill JK, Oliver TH, Powney GD, Fox R, Thomas CD (2019) Habitat availability explains variation in climate-driven range shifts across multiple taxonomic groups. Sci Rep 9:15039
Pöyry J, Luoto M, Heikkinen RK, Kuussaari M, Saarinen K (2009) Species traits explain recent range shifts of Finnish butterflies. Glob Change Biol 15:732–743
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Raven PH, Wagner DL (2021) Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc Nat Acad Sci USA 118:e2002548117
Roy DB, Ploquin EF, Randle Z, Risely K, Botham MS, Middlebrook I, Noble D, Cruickshanks K, Freeman SN, Brereton TM (2015) Comparison of trends in butterfly populations between monitoring schemes. J Insect Conserv 19:313–324
Samways MJ, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kaila L, Kwak ML, Maes D, Mammola S, Noriega JA, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Settele J, Simaika JP, Stork NE, Suhling F, Vorster C, Cardoso P (2020) Solutions for humanity on how to conserve insects. Biol Conserv 242:108427
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarlı D, Ammer C, Bauhus J, Fischer M, Habel JC, Linsenmair KE, Nauss T, Penone C, Prati D, Schall P, Schulze E-D, Vogt J, Wöllauer S, Weisser WW (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst 40:415–436
Soroye P, Newbold T, Kerr J (2020) Climate change contributes to widespread declines among bumble bees across continents. Science 367:685–688
Stenrød M (2015) Long-term trends of pesticides in Norwegian agricultural streams and potential future challenges in northern climate. Acta Agri Scand Sec B 65:199–216
Sterling P, Parsons M (2012) Field guide to the micro-moths of Great Britain and Ireland. British Wildlife Publishing, Gillingham
Svensson I (2006) Nordens vecklare: the nordic tortricidae (Lepidoptera, Tortricidae). Entomologiska Sällskapet, Lund
Svetunkov I (2020) Smooth: forecasting using state space models. R package version 2.5.5. https://CRAN.R-project.org/package=smooth
Therneau T, Atkinson B (2019) Rpart: recursive partitioning and regression trees. R package version 4.1-15. https://CRAN.R-project.org/package=rpart
Thomas CD, Jones TH, Hartley SE (2019) “Insectageddon”: a call for more robust data and rigorous analyses. Glob Change Biol 25:1891–1892
Valtonen A, Hirka A, Szőcs L, Ayres MP, Roininen H, Csóka G (2017) Long-term species loss and homogenization of moth communities in Central Europe. J Anim Ecol 86:730–738
Van Dyck H, Van Strien AJ, Maes D, Van Swaay CAM (2009) Declines in common, widespread butterflies in a landscape under intense human use. Conserv Biol 23:957–965
Van Dyck H, Bonte D, Puls R, Gotthard K, Maes D (2015) The lost generation hypothesis: could climate change drive ectotherms into a developmental trap? Oikos 124:54–61
van Klink R, Bowler DE, Gongalsky KB, Swengel AB, Gentile A, Chase JM (2020) Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368:417
Wagner DL (2020) Insect declines in the anthropocene. Annu Rev Entomol 65:457–480
Wagner DL, Fox R, Salcido DM, Dyer LA (2021) A window to the world of global insect declines: moth biodiversity trends are complex and heterogeneous. Proc Nat Acad Sci USA 118:e2002549117
Warren MS, Hill JK, Thomas JA, Asher J, Fox R, Huntley B, Roy DB, Telfer MG, Jeffcoate S, Harding P, Jeffcoate G, Willis SG, Greatorex-Davies JN, Moss D, Thomas CD (2001) Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414:65–69
Watanabe S (2010) Asymptotic equivalence of bayes cross validation and widely applicable information criterion in singular learning theory. J Mach Learn Res 11:3571–3594
Welti EAR, Roeder KA, De Beurs KM, Joern A, Kaspari M (2020) Nutrient dilution and climate cycles underlie declines in a dominant insect herbivore. Proc Nat Acad Sci USA 117:7271–7275
Zellweger F, De Frenne P, Lenoir J, Vangansbeke P, Verheyen K, Bernhardt-Römermann M, Baeten L, Hédl R, Berki I, Brunet J (2020) Forest microclimate dynamics drive plant responses to warming. Science 368:772–775
Acknowledgements
We thank Otso Ovaskainen and Gleb Tikhonov for a helpful HMSC workshop and timely package updates, and Mahdieh Tourani for suggestions on time-series analyses. J. Fjelddalen helped with geometrid moth identifications. High-performance computing resources were provided by Frederick H. Sheldon and Louisiana State University (LSU HPC). This project was supported by internal funding from the Faculty of Environmental Sciences and Natural Resource Management, Norwegian University of Life Sciences.
Funding
Open access funding provided by Norwegian University of Life Sciences. This project was supported by internal funding from the Faculty of Environmental Sciences and Natural Resource Management, Norwegian University of Life Sciences.
Author information
Authors and Affiliations
Contributions
SK developed and completed the long-term month monitoring; RCB, VS, SK, RJ and AST conceived the ideas for the present study; VS compiled the trait data; RCB developed the analyses, analyzed the data, and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Correspondence and current address: Ryan C. Burner, Upper Midwest Environmental Sciences Center, U.S. Geological Survey, La Crosse, WI, USA
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burner, R.C., Selås, V., Kobro, S. et al. Moth species richness and diversity decline in a 30-year time series in Norway, irrespective of species’ latitudinal range extent and habitat. J Insect Conserv 25, 887–896 (2021). https://doi.org/10.1007/s10841-021-00353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-021-00353-4