Abstract
During 2002–2013, we surveyed butterflies in three types of bogs (pristine but naturally fragmented). Of the 75 bogs surveyed, we established 29 bogs and 5 bog roadsides as long-term sites visited annually for 9–15 years. We studied patterns of ten butterfly species’ flight periods, annual variation, trend in abundance over time, and abundance with respect to climatic variables. First observed date per year varied more for spring than summer species. Jutta arctic Oeneis jutta varied between dramatically high numbers in odd years and low numbers in even years in northeast Wisconsin. Elsewhere, Jutta arctic varied less between odd and even years, but muskegs had higher numbers in even than odd years, significantly so in north central Wisconsin. The most abundant bog affiliate (tyrphophile), brown elfin Callophrys augustinus, exhibited cyclic abundance over a 4–5 year period. The other species varied greatly in abundance among years but not in as distinctive annual patterns. The most northern specialist (tyrphobiont), purplish fritillary Boloria montinus, declined strongly. Its abundance significantly related to higher precipitation but not to temperature. Population trends for the seven other tyrphobionts did not relate to how southerly their ranges are. Trends in roadsides were less favorable than in bogs. Butterfly abundance had more significant correlations to climate variables related to moisture than to temperature. Based on abundance relationships to climate, a majority of the study species allied as dry steppe species (increasing in warmer, drier periods) and a minority as wetland species (increasing in cooler, wetter periods). The overriding factors determining the future of this butterfly fauna appear to be habitat degradation and potential climate change beyond the range of variation typically experienced in this region between glaciations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In temperate areas of North America and Europe, bog (peatland) vegetation is naturally rare and isolated, forming a low proportion of the natural landscape (Spitzer et al. 1999; Spitzer and Danks 2006; Whitehouse 2006; Whitehouse et al. 2008). In Wisconsin, peatlands occur primarily in central and northern areas (Curtis 1959). Prior to European settlement, peatlands occupied <1 % of the Wisconsin landscape (Hoffman 2002). Most of that vegetation is still extant, with only 9 % loss, and much of what is left, especially in northern Wisconsin, is relatively undegraded. Primary human impacts are roads, ditches, and logging along the margins; adjacent lands are more affected by timber harvesting, agriculture, off-road vehicles, and urbanization (pers. obs.; Wright et al. 1992; Epstein et al. 1997). Conversion to cranberry agriculture and peat harvesting have occurred more in central Wisconsin bogs (Curtis 1959). By contrast, in Europe bog vegetation is much destroyed and degraded by human activities, and its associated butterfly species are of high conservation concern (Spencer and Collins 2008; Turlure et al. 2009). The four bog-related vegetation types ranked highest in proportion of threatened butterfly species of their typical faunas (van Swaay et al. 2006).
Wisconsin bogs present the opportunity to study how localized butterfly populations function in relatively undisturbed sites in a naturally fragmented landscape. Prior studies (Nekola 1998; Nekola and Kraft 2002; Swengel and Swengel 2010, 2011; Johnson 2011) described patterns of bog butterfly population incidence and abundance. In this paper, we extend that work by examining change in bog butterfly abundance over time in our Wisconsin bog butterfly surveys during 2002–2013. We analyze time-series of population abundance in annually surveyed sites to describe the range of variation among years (patterns of fluctuation), relationships to climatic factors, and population trend (correlations with year) by bog type and subregion. These results should be useful for application to conservation of bog butterflies where they are vulnerable, and for understanding vulnerable butterflies in other fragmented vegetations.
Methods
Study region
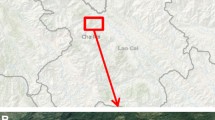
We surveyed 75 bogs in the study region (Fig. 1). They were scattered across an area 367 km east–west by 169 km north–south (45.33–46.86°N, 88.21–92.56°W) spanning the entire breadth of northern Wisconsin (Swengel and Swengel 2010, 2011). At 20 of these sites, we also surveyed the lowland (wetland) roadside ditch through or adjacent to the bog. All sites could not be visited each year but most were visited more than once both within and among years. We consistently surveyed 29 bogs and 5 roadsides each year from the starting year (1999–2005) through 2013. We classified our study sites (Swengel and Swengel 2013) according to Nekola’s (1998) categories: (1) muskeg (black spruce Picea mariana-cottongrass Eriophorum spissum-wiregrass Carex oligosperma-Sphagnum savanna similar in elevation to surrounding uplands), (2) kettlehole (Sphagnum-leatherleaf Chamaedaphne calyculata mats, often floating on lakes or sunk in depressions much lower than the surrounding landscape), and (3) coastal peatland (tamarack Larix laricina-sedge, especially Carex lasiocarpa, mats with ridges of muskeg-like vegetation in estuaries along the Lake Superior coast).
Map (right) showing subregions where 29 long-term study bogs were located in northern Wisconsin (90 km between nearest northeast and north central muskegs; 117 km between nearest north central and northwest muskegs). Forty-six other bogs surveyed less often were north of the dashed line. Also shown are barrens surveyed in central Wisconsin as described in Swengel and Swengel (2010), with results presented in Online Resource 11
Surveys
We conducted most formal transect surveys in bogs during 2002–2013, with a few conducted in years before that (Swengel and Swengel 2010, 2011). Beginning in 2002, we surveyed in a rotation through the western, central, and eastern sections of the bog study region (Fig. 1), trying to cover one section per weekend, or more if a section was missed the previous weekend and/or if time allowed. We missed an occasional weekend per year due to weather or another commitment. Surveys occurred between 25 March and 12 September, usually from April to early May through mid-August.
We walked transect surveys along similar routes per visit to a site at a slow pace (about 2 km/h) on parallel routes 5–10 m apart (as described in Swengel and Swengel 2010, 2011), similar to Pollard (1977). We counted all adult butterflies observed ahead and to the sides, to the limit an individual could be identified, possibly with binoculars after detection, and tracked. Surveys occurred during a wide range of times of day and weather, occasionally in light drizzle so long as butterfly activity was apparent, but not in continuous rain.
Analyses
We analyzed 10 study species (Table 1), classified into two categories that correspond approximately to those (in parentheses) described by Spitzer and Danks (2006): (1) bog specialist (tyrphobiont)—restricted or nearly so to peatlands (all eight species known in Wisconsin), and (2) bog affiliate (tyrphophile)—breeding in bogs as well as other vegetations; limited to species of north temperate or boreal affinity (two species). These are Nekola’s (1998) ten peatland-obligate study species that complete their entire life cycle in bogs. As in Nekola and Kraft (2002), we identified the flight period per species per year by the first and last date we detected it across the study region.
Our population abundance index is the peak survey count per site per year, standardized to survey time to create an observation rate (relative abundance) per hour per site. We surveyed sites multiple times per year both to verify the timing of the main flight period and to survey different target species. However, a “collated” index (e.g. sum of weekly counts throughout a species’ flight period in a year) was not possible because the number of visits per flight period varied both among sites and among years. We avoided pseudoreplication (counting the same individual on more than one survey) because our population indices all contain only a single sampling of individuals per generation per species at each site. One survey during the main flight period has been adequate for producing representative indices for comparisons of relative abundance within and among sites (Thomas 1983; Swengel and Swengel 2005; Schlicht et al. 2009). We assembled time series for sites surveyed consistently for a species annually from 2002 to 2005 through 2013.
We obtained climate data for winter (December–February), spring (March–May), summer (June to August), the growing season (April–September), and year from the Wisconsin State Climatology Office (2013). Our northeast, north central, and northwest (including muskegs, kettleholes, and coastal peatlands) subregions correspond to the same subregions in this climate dataset. We analyzed seasonal temperature, precipitation, and season-long snowfall total (from the prior year’s fall to the current year’s spring), and the Palmer Drought Severity Index, which becomes more positive in floods and more negative in droughts. This index uses temperature and precipitation data to evaluate potential evapotranspiration to measure environmental dryness [cumulative departure in surface water balance (Dai et al. 2014)]. The climate data were available up to 2011, except 2010 for snowfall. We matched climate data to butterfly abundance by subregion and year, and correlated butterfly relative abundance at the scale of the site time series to climate for up to one year after the timing of the climate variable.
We identified our first observed date (FOD) each year that we found each species at any bog site anywhere in the bog study region (Fig. 1). It was not possible to subdivide these dates by subregion because the interval between visits within a subregion was usually more than a week. We calculated the mean FOD for each species during 2002–2013, and correlated those means to the standard deviation (SD) for each mean FOD and to the difference in days between the earliest and latest FOD for each species.
Analysis was done with ABstat 7.20 software (1994 Anderson-Bell Corp., Parker, Colorado). Statistical significance was set at two-tailed P < 0.05. Since significant results occurred much more frequently than expected due to Type I statistical errors, we did not lower the critical P value further, as far more Type II errors (biologically meaningful patterns lacking statistical significance) would be created than Type I errors eliminated. We chose to use non-parametric tests because they do not require data to be distributed normally. All correlations were done with the Spearman rank correlation. We used the Mann–Whitney U test to test for significant differences in relative abundance of Jutta arctic between even and odd years. As reviewed in Swengel and Swengel (2013), Jutta arctic varies among parts of its range as to whether adults are found primarily or only in even or odd years, or readily in both even and odd years.
We performed analyses at the scale of the individual species at the individual site per year. We did not average or otherwise collate the survey results for a species in a year across sites prior to analysis for two reasons. First, the start year for the time series varied by site. Second, we wanted to retain local variation in the statistical tests. Because butterfly survey counts can lack a normal distribution, the mean may be skewed toward the high abundance of a few sites when most other sites had few or no individuals found. By analyzing at the scale of the site, rather than the region, each site was represented equally in statistical tests.
Results
First observed date (FOD)
FOD was more variable for spring than summer butterflies (Table 2). The mean FOD for each species during 2002–2013 correlated highly negatively with the SD of that date (r = −0.770, N = 10 species, P < 0.01) and span of that date (difference in days between earliest and latest FOD for a species over 12 years; r = −0.766, N = 10 species, P < 0.01). All correlations of FOD with spring temperature were negative (Table 2), significantly so for four species. One spring species, red-disked alpine, had a significant negative correlation of abundance with FOD (Table 3). Two spring and one summer species had significant positive correlations between FOD and the following year’s abundance (brown elfin, Frigga fritillary, common ringlet) (Table 3).
Annual variation and population trends
Brown elfin exhibited remarkably regular, possibly cyclic variation with low abundance synchronized over the entire study region (Fig. 2, Online Resource 1). Abundance patterns in each subregion for the other study species are provided in Online Resources 2–10. Jutta arctic showed dramatic consistent variation in abundance between even and odd years in Northeast (Online Resource 6) with odd years significantly higher than even (Fig. 3). This even–odd variation was not obvious in the other subregions (Online Resource 6) but was nonetheless significantly different in North Central in the opposite direction (Fig. 3). The other study species varied greatly in abundance among years but not in as distinctive patterns (Online Resources 1–5, 7–10).
Significant trends of abundance over time (Table 4) were positive for spring butterflies (brown elfin, Freija fritillary, red-disked alpine) and negative for summer butterflies (common ringlet, bog copper, purplish fritillary) with the exception of the Dorcas copper, which was positive. However, common ringlet has been broadly increasing in abundance farther south in central Wisconsin barrens (Online Resource 11). Within species, the trend in roadsides was usually less favorable than in bogs in the same region (common ringlet, Dorcas copper, bog copper in northwest but not north central, purplish fritillary) (Table 4).
Climate
All ten study species had significant correlations with climate variables (Table 5), more so with precipitation than temperature. All correlations to winter climate variables indicated increasing abundance with coolness (4 species) and/or dryness (6 species). In correlations to current-year spring climate variables, four species favored dryness and/or warmth. However, brown elfin (the earliest spring-flying species) favored coolness, and the two latest summer-flying species favored wetness. Correlations of summer-flying species to current-year summer variables all related to moisture. The species that had higher current-year abundance with earlier first observed dates (Table 3) was a spring flier that favored a dry winter and spring (Table 5).
For some species, lag effects of climate variables on next year’s butterfly abundance were similar to those matching current-year climate and abundance (Table 5). Examples include Jutta arctic (warmth and dryness), and bog copper and purplish fritillary (wetness). Other species showed seasonal variation in temperature or precipitation preferences. For example, brown elfin favored cool and dry in the current winter and spring but warm and dry in the previous summer. Freija fritillary favored wetness in the previous summer but otherwise dryness in both the current and previous summer. Frigga fritillary favored warmth in the current spring but coolness in the previous year. Bog fritillary favored summer wetness for the current year’s adult abundance but dryness for next year’s abundance.
Three species (bog fritillary, bog copper, purplish fritillary) consistently showed an affinity for moistness (Table 5), although bog fritillary favored dryness in the previous year. Within a site, bog fritillary abundance covaried strongly with bog copper abundance later in the same year, and bog copper abundance covaried strongly with the following year’s bog fritillary abundance (Table 6). Bog fritillary and bog copper were the two study species more abundant in kettleholes and coastal peatlands than muskegs (Table 1). All bog copper’s consistent preferences for moistness occurred in muskegs (Table 5). Bog fritillary had no significant relationships to climate variables in muskegs. Bog copper had no significant climate correlations in kettleholes and coastal peatlands nor did bog fritillary in coastal peatlands, even though they dramatically varied in abundance there. Six of the other seven species consistently favored dryness and drought (Table 5), although varying in whether they also favored coolness or warmth. Results for the seventh species (Dorcas copper) were equivocal, since it favored cool and dry in the current-year winter, but warm and wet in the prior year’s growing season.
Discussion
First observed date (FOD)
Although FOD can have some biases due to variation in sites and sampling intensity among years (van Strien et al. 2008), our study is less prone to these issues because sampling effort and sites were relatively similar among years, and observers were always the same. Furthermore, the consistent negative correlation of FOD and spring temperature (Table 2) was expected from the literature relating variation in butterfly flight periods to variation among years in seasonal development (examples in Sparks and Carey 1995; Sparks and Yates 1997; Forister and Shapiro 2003; Polgar et al. 2013), and provides a validation of the FODs. But the correlations of FOD to some species’ abundance (Table 3) also support that FOD relates both to phenology and butterfly abundance (van Strien et al. 2008).
Spring butterflies consistently varied more in FOD than summer butterflies (Table 2), as also noted in other butterfly faunas (Forister and Shapiro 2003; Polgar et al. 2013). The effect on adult butterflies could result from butterflies being easier to detect, but not more abundant, in warmer weather, which might explain why this effect was more evident for spring than summer butterflies. Adult butterflies might be more apparent in warm springs because of the greater amount of time spent flying (Cormont et al. 2011). The lag effect of FOD on next year’s adult abundance (Table 3) might result primarily from effects on immatures. Climatic influences on abundance may conflict between the current year and the following year, i.e., on different life stages. For example, in Europe the negative effect of winter warmth on larval survival of bog fritillary far outweighed the positive effects of spring and summer warmth on egg, pre-diapause larval, and pupal survival, and number of eggs laid (Schtickzelle and Baguette 2004; Radchuk et al. 2013).
Annual variation
Dramatic variation in abundance usually appeared to correspond to the frequently reported fluctuations in butterfly abundance related to climatic variation (examples in Dennis 1993; Pollard and Yates 1993; Roy et al. 2001; Warren et al. 2001). This acts separately on individual populations yet can produce remarkably synchronized variation in abundance among populations over much larger spatial scales than individuals in these populations typically disperse (Sutcliffe et al. 1996). However, even though brown elfin exhibited significant relationships to climate (Table 5), its time series also showed a consistent seemingly cyclic variation (Fig. 2, Online Resource 1), suggestive of density-dependent population regulation such as reported for holly blue Celastrina argiolus in Britain (Thomas et al. 2011: Fig. 4). The brown elfin’s larval host plants are heaths (Ericaceae) (Opler and Krizek 1984; Nielsen 1999). These plants appear abundant in muskegs, less so in kettleholes and coastal peatlands, although larval breeding requirements may be more limiting than host plant abundance as reported for other butterflies (Turlure et al. 2010; Thomas et al. 2011).
Jutta arctic exhibited dramatically consistent higher abundance in odd than even years in northeast (Fig. 3, Online Resource 6). In the adjacent north central subregion, Jutta arctic showed less dramatic but significantly higher abundance in even years (Fig. 3) but this did not appear as regular and predictable as the pattern in the Northeast. This shift in even/odd year abundance patterns among adjacent subregions is consistent with reports from elsewhere in the species’ range, as reviewed in Swengel and Swengel (2013). This species is reported to have a two-year life cycle (Scott 1986).
Population trends
Within species, the trend in roadsides was usually less favorable than in bogs in the same region (common ringlet, Dorcas copper, bog copper in northwest but not north central, purplish fritillary) (Table 4). This suggests the negative influence of human disturbance in the landscape, since bog sites (away from roadsides) had fewer human-caused degradations, such as the trenching, scarification, and re-grading we observed in roadsides.
The most northerly species (purplish fritillary) had a strongly negative population trend (Table 4), consistent with numerous others’ reports of northern species retreating uphill or northward (e.g., Parmesan 1996; Forister et al. 2010; Breed et al. 2013). Nekola and Kraft (2002) expressed concern about the long-term future in Wisconsin for the purplish fritillary. It is in a species complex, or conspecific, with arctic fritillary Boloria chariclea (Opler and Krizek 1984; Scott 1986; Johnson 2011), which in Europe is a butterfly likely to have the most severe range contractions with climatic warming (Settele et al. 2008; Heikkenen et al. 2010).
But southerliness of range otherwise did not correspond to trends (Table 4). Bog copper has the southernmost range of the tyrphobionts (Glassberg 1999) but had a negative trend. Freija fritillary and red-disked alpine are relatively northern species but had positive trends. While the significant trend for common ringlet in the bog study region was negative, this species was increasing in abundance 120–300 km farther south in central Wisconsin barrens (Online Resource 11). Over the last 55 years, this species has appeared to be colonizing the state from the northwest (Ebner 1970).
Thus, some of the trends in bogs (Table 4) may relate not to temperature (as assumed in a north–south analysis) but to precipitation, or changes in habitat characteristics (e.g. disturbances or drainage at bog edges). Furthermore, these species may tolerate climatic conditions outside of their observed geographic ranges. Warren et al. (2001) noted that the ranges of many British butterfly species appear limited by factors other than climate, since they are not occupying all areas that are climatically suitable. Unexpected outcomes such as downhill expansion of some high arctic butterflies in a Swedish arctic alpine national park may result from the complexity of seasonal variation in both temperature and precipitation (Franzén and Öckinger 2012). The large variation in annual abundance of bog butterflies in this study, and uncertainty about longer-term effects of climate, highlight the value of longer monitoring periods than in our study (Thomas et al. 2002).
Climate
Since this analysis tested for one kind of pattern (correlation) (Table 5), it would not detect many other kinds of climatic influences such as threshold or non-linear effects or multivariate interactions. Caution should be used in any application of these results to predict future bog butterfly responses to climatic variation.
This analysis did not produce consistent relationships such as southern species tolerating warmth more than northern species, or tyrphobionts preferring wetness and coolness (Table 5). The one season where all significant relationships indicated the same preference (cool, dry) was winter, which suggests that these conditions improved overwintering immature survival. Otherwise, in spring and summer, the species varied as to whether they favored cool or warm, moist or dry.
However, this analysis of butterfly abundance more often produced significant results related to moisture than to temperature (Table 5). Most of the study species clearly categorized into two guilds (regardless of range relative to Wisconsin), with a majority classifying as dry steppe species and the remaining minority (bog fritillary, bog copper, purplish fritillary) as wetland species. Only one species (Dorcas copper) was unclear how to classify in this regard because of conflicting climatic results between current-year and lag-year effects on abundance.
The relationships of the study species’ abundance to moisture (Table 5) consistently corresponded to their differences in abundances by bog type (Table 1, Online Resource 1; Nekola 1998; Swengel and Swengel 2011). Kettleholes (some of which float on a lake) and coastal peatlands are more consistently moist, while muskegs (or parts of muskegs) can be drier (Nekola 1998). Furthermore, differences in the species’ climatic preferences by bog type were also generally consistent with the differences in moistness among the bog types. Two of the three species in the wetland guild (bog fritillary, bog copper) were far more abundant in the wetter bog types (kettlehole and coastal peatlands). Both had more favorable trends in coastal peatlands (with a more maritime climate) than inland kettleholes (Table 4). All of bog copper’s significant preferences for moister weather occurred in the drier bog type (muskeg) (Table 5). Bog copper showed no significant climatic correlations in kettleholes and coastal peatlands, nor did bog fritillary in coastal peatlands, even though they varied dramatically in abundance there. However, bog fritillary did favor dryness in kettleholes as a lag-year effect. Climatic relationships might become apparent for these species in coastal peatlands with climate data restricted to weather stations along the Lake Superior coast, which is far cooler in the growing season than nearby inland locations (Curtis 1959; Zimmerman 1991).
The third species (purplish fritillary) in this wetland guild only occurred in muskegs in northwest. Nekola (1998) identified this species as one of five members of a high arctic dry sedge-heath guild. Purplish fritillary’s preference for moistness may serve as a temperature buffer, for this is the study species with the northernmost southern edge to its range (Opler and Krizek 1984; Glassberg 1999). The closely related B. titania in Switzerland likewise preferred a locally higher proportion of wetland, as well as more trees, which may also serve as a climatic buffer (Cozzi et al. 2008).
All species in the steppe guild occurred more abundantly or only in muskegs (Table 1). Three of the five taxa in Nekola’s (1998) high arctic guild (freija and frigga fritillaries, red-disked alpine) are in the steppe guild identified here. Dorcas copper is also in Nekola’s (1998) high arctic guild but is not clearly classified in our analysis of abundance related to climate data. Both tyrphophile study species are in the steppe guild and occurred in all three bog types. They also occur widely in non-wetland habitats: brown elfin in barrens with its ericaceous hosts and ringlets in grasslands, including barrens (Online Resource 11) (Opler and Krizek 1984; Glassberg 1999).
Five species showed conflicts among seasons in temperature or precipitation effects on abundance (Table 5), possibly related to which life stage was affected by the climatic variable (WallisDeVries and van Swaay 2006; Settele et al. 2008; Cormont et al. 2011; Radchuk et al. 2013). For example, bog fritillary favored summer wetness for the current year’s adult abundance but dryness for next year’s abundance, possibly due to how that moisture affects larvae. Schtickzelle and Baguette (2004) found complex variation in bog fritillary population response to climate depending on the season measured (including a preference for cool winters as found here), with an overall negative effect of warm temperatures.
Conservation implications
Tyrphobionts do not form a single guild and do not respond to vegetative factors similarly, as evidenced by significant differences in abundance by bog type (Nekola 1998; Swengel and Swengel 2011). Based on their abundance relationships to climatic variables, most tyrphobionts in this study allied as dry steppe species, while two tyrphobionts with relatively more southerly ranges allied as wetland species. Half of the steppe guild species had positive population trends, while two out of three wetland guild species and no steppe species significantly declined. The species did not respond consistently to temperature in relation to the southerliness of their range. Many tyrphobionts favored warmer growing season conditions and earlier phenologies. Thus, climate did not appear directly limiting on the ranges of these animal populations. They have experienced warmer climate since the last glaciation (e.g., the hypsithermal) (Pielou 1991; Ashworth 2001; Mauquoy and Yeloff 2008) as well as extreme variation in climate, as evidenced in the variation among years in first observed date. However, there may be a lag in butterfly population responses to climate change (Menéndez et al. 2006).
The overriding factors determining the future of this bog butterfly fauna appear to be habitat degradation and potential climate change beyond the range of variation typically experienced in this region between glaciations. The benefit of protecting bogs from degradation is evident in the poorer butterfly abundance trends in roadsides in this study. Elsewhere in the world, habitat destruction and alteration of both the bogs and the surrounding matrix threaten many bog butterfly populations (van Swaay et al. 2006; Spencer and Collins 2008; Turlure et al. 2009; Weking et al. 2013). Degraded or late-succession bogs have fewer hummocks, which are essential thermal refuges (Turlure et al. 2010). Thus, stopping and reversing habitat deterioration are climate change mitigations (Essl et al. 2012), as demonstrated more generally in Thomas et al. (2012). Maintaining a diversity of vegetative structures is necessary to allow larvae to thermoregulate successfully (Turlure et al. 2011). However, some climate change models indicate that the tipping point could be reached when peatland vegetation would no longer be able to persist in more southerly areas such as our study region (Mauquoy and Yeloff 2008; Essl et al. 2012). In that scenario, it would be unlikely that the conditions these species require would continue to exist.
References
ABstat 7.20 (1994) Anderson-Bell Corp., Parker
Ashworth AC (2001) Perspectives on quaternary beetles and climate change. In: Gerhard LC, Harrison WE, Hanson BM (eds) Geological perspectives of global climate change. American Association of Petroleum Geologists Studies in Geology #47, Tulsa, Oklahoma, pp 153–168
Breed GA, Stichter S, Crone E (2013) Climate-driven changes in northeastern US butterfly communities. Nat Clim Change 3:142–145
Cormont A, Malinowska AH, Kostenko O et al (2011) Effect of local weather on butterfly flight behaviour, movement, and colonization: significance for dispersal under climate change. Biodivers Conserv 20:483–503
Cozzi G, Müller CB, Krauss J (2008) How do local habitat management and landscape structure at different spatial scales affect fritillary butterfly distribution on fragmented wetlands? Landsc Ecol 23:269–283
Curtis JT (1959) The vegetation of Wisconsin. University of Wisconsin Press, Madison
Dai A, National Center for Atmospheric Research Staff (eds) (2014) The Climate Data Guide: Palmer Drought Severity Index (PDSI). https://climatedataguide.ucar.edu/climate-data/palmer-drought-severity-index-pdsi. Last modified 21 Mar 2014. Accessed 25 Aug 2014
Dennis RLH (1993) Butterflies and climate change. Manchester University Press, Manchester
Ebner JA (1970) The butterflies of Wisconsin. Milwaukee Public Museum Popular Science Handbook No. 12, Milwaukee, Wisconsin, USA
Epstein EJ, Judziewicz EJ, Smith WA (1997) Wisconsin’s Lake Superior coastal wetlands evaluation, including other selected natural features of the Lake Superior Basin. PUB ER-095 99 of the Bureau of End. Res., Wisc. Dept. Nat. Res., Madison http://dnr.wi.gov/topic/wetlands/cw/pdfs/superior/superior_text.pdf. Accessed 13 Nov 2012
Essl F, Dullinger S, Moser D, Rabitsch W, Kleinbauer I (2012) Vulnerability of mires under climate change: implications for nature conservation and climate change adaptation. Biodivers Conserv 21:655–669
Forister ML, Shapiro AM (2003) Climatic trends and advancing spring flight of butterflies in lowland California. Glob Clim Change 9:1130–1135
Forister ML, McCall AC, Sanders NJ et al (2010) Compounded effects of climate change and habitat shift patterns of butterfly diversity. Proc Natl Acad Sci USA 107:2088–2092
Franzén M, Öckinger E (2012) Climate-driven changes in pollinator assemblages during the last 60 years in an arctic mountain region in northern Scandinavia. J Insect Conserv 16:227–238
Glassberg J (1999) Butterflies through binoculars: the East. Oxford University Press, New York
Heikkenen RK, Luoto M, Leikola N, Pöyry J, Settele J, Kudrna O, Marmion M, Fronzek S, Thuiller W (2010) Assessing the vulnerability of European butterflies to climate change using multiple criteria. Biodiv Conserv 19:695–703
Hoffman RM (2002) Wisconsin’s Natural Communities: how to recognize them, where to find them. University of Wisconsin Press, Madison
Johnson KE (2011) Distributions, habitats, and natural histories of Boloria (Lepidoptera: Nymphalidae) inhabiting central North American peatlands, emphasizing the Great Lakes region. MS thesis, University of Wisconsin, Madison
Mauquoy D, Yeloff D (2008) Raised peat bog development and possible responses to environmental changes during the mid- to late-Holocene. Can the palaeoecological record be used to predict the nature and response of raised peat bogs to future climate change? Biodivers Conserv 17:2139–2151
Menéndez R, González Megías A, Hill JK et al (2006) Species richness changes lag behind climate change. Proc R Soc B 273:1465–1470
Nekola JC (1998) Butterfly (Lepidoptera: Lycaenidae, Nymphalidae, and Satyridae) faunas of three peatland habitat types in the Lake Superior drainage basin of Wisconsin. Great Lakes Entomol 31:27–38
Nekola JC, Kraft CE (2002) Spatial constraint of peatland butterfly occurrences within a heterogenous landscape. Oecologia 130:53–61
Nielsen M (1999) Michigan butterflies and skippers. Michigan State University Extension, East Lansing
Opler PA, Krizek GO (1984) Butterflies east of the Great Plains. Johns Hopkins University Press, Baltimore
Parmesan C (1996) Climate and species’ range. Nature 382:765–767
Pielou EC (1991) After the ice age: the return of life to glaciated North America. University of Chicago Press, Chicago
Polgar CA, Primack RB, Williams EH, Stichter S, Hitchcock C (2013) Climate effects on the flight period of Lycaenid butterflies in Massachusetts. Biol Conserv 160:25–31
Pollard E (1977) A method for assessing changes in abundance of butterflies. Biol Conserv 12:115–133
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation. Chapman & Hall, London
Radchuk V, Turlure C, Schtickzelle N (2013) Each life stage matters: the importance of assessing the response to climate change over the complete life cycle in butterflies. J Anim Ecol 82:275–285
Roy DB, Rothery P, Moss D, Pollard E, Thomas JA (2001) Butterfly numbers and weather: predicting historical trends in abundance and the future effects of climate change. J Anim Ecol 70:201–217
Schlicht D, Swengel A, Swengel S (2009) Meta-analysis of survey data to assess trends of prairie butterflies in Minnesota, USA during 1979–2005. J Insect Conserv 13:429–447
Schtickzelle N, Baguette M (2004) Metapopulation viability analysis of the bog fritillary butterfly using RAMAS/GIS. Oikos 104:277–290
Scott JA (1986) The butterflies of North America. Stanford University Press, Stanford
Settele J, Kudrna O, Harpke A, Kühn I, van Swaay C, Verovnik R, Warren M, Wiemers M, Hanspach J, Hickler T, Kühn E, Van Halder I, Veling K, Vliegenthart A, Wynhoff I, Schweiger O (2008) Climatic risk atlas of European butterflies. Pensoft Publishers, Sofia
Sparks TH, Carey PD (1995) The responses of species to climate over two centuries: an analysis of the Marsham phenological record, 1736–1947. J Ecol 83:321–329
Sparks TH, Yates TJ (1997) The effect of spring temperature on the appearance dates of British butterflies 1883–1993. Ecography 20:368–374
Spencer S, Collins S (2008) Reversing the decline in butterflies and moths across Europe—the importance of particular farming practices and the implications for CAP reform. www.birdlife.eu/eu/pdfs/BCEurope_CAPreformpaperFeb08.pdf. Accessed 15 Jan 2010
Spitzer K, Danks HV (2006) Insect biodiversity of boreal peat bogs. Annu Rev Entomol 51:137–161
Spitzer K, Bezdĕk A, Jaroš J (1999) Ecological succession of a relict Central European peat bog and variability of its insect biodiversity. J Insect Conserv 3:97–106
Sutcliffe OL, Thomas CD, Moss D et al (1996) Spatial synchrony and asynchrony in butterfly population dynamics. J Anim Ecol 65:85–95
Swengel AB, Swengel SR (2005) Long-term population monitoring of the Karner Blue (Lepidoptera: Lycaenidae) in Wisconsin, 1990–2004. Great Lakes Entomol 38:107–134
Swengel AB, Swengel SR (2010) The butterfly fauna of Wisconsin bogs: lessons for conservation. Biodivers Conserv 19:3565–3581
Swengel AB, Swengel SR (2011) High and dry or sunk and dunked: lessons for tallgrass prairies from quaking bogs. J Insect Conserv 15:165–178
Swengel SR, Swengel AB (2013) Jutta arctic (Oeneis jutta) (Lepidoptera: Nymphalidae) populations in central and northern Wisconsin: localized butterfly populations in a naturally fragmented landscape. Great Lakes Entomol 46:174–192
Thomas JA (1983) A quick method for estimating butterfly numbers during surveys. Biol Conserv 27:195–211
Thomas CD, Wilson RJ, Lewis OT (2002) Short-term studies underestimate 30-generation changes in a butterfly metapopulation. Proc R Soc Lond B 269:563–569
Thomas JA, Simcox DJ, Hovestadt T (2011) Evidence based conservation of butterflies. J Insect Conserv 15:241–258
Thomas CD, Gillingham PK, Bradbury RB et al (2012) Protected areas facilitate species’ range expansions. Proc Natl Acad Sci USA 109:14063–14068
Turlure DH, Van Dyck N, Schtickzelle N, Baguette M (2009) Resource-based habitat definition, niche overlap and conservation of two sympatric glacial relict butterflies. Oikos 118:950–960
Turlure C, Choutt J, Baguette M, Van Dyck H (2010) Microclimatic buffering and resource-based habitat in a glacial relict butterfly: significance for conservation under climate change. Glob Change Biol 16:1863–1893
Turlure C, Radchuk V, Baguette M, Van Dyck H, Schtickzelle N (2011) On the significance of structural vegetation elements for caterpillar thermoregulation in two peat bog butterflies: Boloria eunomia and B. aquilonaris. J Therm Biol 36:173–180
van Strien AJ, Plantenga WF, Soldaat LL, van Swaay CAM, WallisDeVries MF (2008) Bias in phenology assessments based on first appearance data of butterflies. Oecologia 156:227–235
van Swaay CAM, Warren MS, Loïs G (2006) Biotope use and trends of European butterflies. J Insect Conserv 10:189–209
WallisDeVries MF, van Swaay CAM (2006) Global warming and excess nitrogen may induce butterfly decline by microclimatic cooling. Glob Change Biol 12:1620–1626
Warren MS, Hill JK, Thomas JA et al (2001) Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414:65–69
Weking S, Hermann G, Fartmann T (2013) Effects of mire type, land use and climate on a strongly declining wetland butterfly. J Insect Conserv 17:1081–1091
Whitehouse NJ (2006) What can forest managers learn from research on fossil insects? Linking forest ecological history, biodiversity and management. In: Grove SJ, Janula JL (eds) Insect biodiversity and dead wood: proceedings of a symposium for the 22nd international congress of entomology. Gen. Tech. Rep. SRS-93. USDA Forest Service, Southern Research Station, Asheville, NC, pp 30–41
Whitehouse NJ, Langdon PG, Bustin R, Galsworthy S (2008) Fossil insects and ecosystem dynamics in wetlands: implications for biodiversity and conservation. Biodiv Conserv 17:2055–2078
Wisconsin State Climatology Office (2013) http://www.aos.wisc.edu/~sco/clim-history/data-portal.html. Accessed 30 Sep and 1 Oct 2013
Wright HE Jr, Coffin BA, Aaseng NA (eds) (1992) The patterned peatlands of Minnesota. University of Minnesota Press, Minneapolis
Zimmerman JH (1991) The landscape and the birds. In: Robbins SD Jr (ed) Wisconsin Birdlife. University of Wisconsin Press, Madison, pp 35–90
Acknowledgments
We greatly appreciate Mrs. Sandra McKibben and Drs. William and Elsa Boyce for funding our bog surveys. We thank Jeff Nekola, Kyle Johnson, and Les Ferge for generously sharing tips and site locations. We thank two anonymous reviewers for numerous helpful comments that greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10841_2014_9731_MOESM10_ESM.pdf
Online Resource 10 Mean bog copper individuals/hr for each bog subregion. Kettlehole results are scaled to the right y-axis (PDF 174 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Swengel, S.R., Swengel, A.B. Assessing abundance patterns of specialized bog butterflies over 12 years in northern Wisconsin USA. J Insect Conserv 19, 293–304 (2015). https://doi.org/10.1007/s10841-014-9731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9731-8