Abstract
Background
Age-stratified analyses of atrial fibrillation (AF) patients undergoing percutaneous left atrial appendage occlusion (LAAO) are limited. The purpose of current study was to compare in-hospital outcomes in elderly AF patients (age > 80 years) to a relatively younger cohort (age £ 80 years) after LAAO.
Methods
Data were extracted from National Inpatient Sample for calendar years 2015–2018. LAAO device implantations were identified on the basis of International Classification of Diseases, 9th and 10th Revision, Clinical Modification codes of 37.90 and 02L73DK. The outcomes assessed in our study included complications, inpatient mortality, and resource utilization with LAAO.
Results
A total of 36,065 LAAO recipients were included in the final analysis, of which 34.6% (n=12,475) were performed on elderly AF patients. Elderly AF patients had a higher prevalence of major complications (6.7% vs. 5.7%, p < 0.01) and mortality (0.4% vs. 0.1%, p < 0.01) after LAAO device implantation in the crude analysis. After multivariate adjustment of potential confounders, age > 80 years was associated with increased risk of inpatient mortality (adjusted odds ratio [aOR] 4.439, 95% confidence interval [CI] 2.391–8.239) but not major complications (aOR 1.084, 95% CI 0.971–1.211), prolonged length of stay (aOR 0.943, 95% CI 0.88–1.101), or increased hospitalization costs (aOR 0.909, 95% CI 0.865–0.955).
Conclusion
Over 1 in 3 LAAO device implantations occurred in elderly AF patients. After adjusting for potential confounding variables, advanced age was associated with inpatient mortality, but not with other LAAO procedural–related outcomes including major complications, prolonged length of stay, or increased hospitalization costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The prevalence of atrial fibrillation (AF) is projected to increase in the United States (US) largely due to advanced age and concomitant co-morbidities that perpetuate this cardiac dysrhythmia [1, 2]. AF is associated with heightened stroke risk and AF-related strokes tend to be more disabling especially in elderly patients [3, 4]. Additionally, elderly patients have an increased risk of major bleeding when oral anticoagulants are utilized for mitigation of stroke risk in such patients [5, 6]. More recently, left atrial appendage occlusion (LAAO) using a Watchman device has shown promise in reducing the stroke risk as an alternative to OAC therapy [7,8,9]. LAAO devices can be deemed favorable in elderly patients when compared to OAC therapy due to improved bleeding risk profile. Unfortunately, the landmark randomized trials evaluating the safety and efficacy of LAAO using a Watchman device have limited participation of patients > 80 years old [7, 8]. Additionally, the few retrospective studies evaluating outcomes after LAAO implantation in older patients have shown conflicting results and were not thoroughly adjusted for baseline co-morbidities that commonly occur in this patient population group and could confound such an association with adverse events [10,11,12]. The aim of the present study was to assess complications and inpatient outcomes while accounting for comorbidities associated with age in elderly AF (> 80 years) patients compared to a younger aged cohort (≤ 80 years) after LAAO implantation.
2 Methods
2.1 Data source
Data from National Inpatient Sample (NIS) was used for the purpose of our current study. We analyzed the NIS database from years 2015 to 2018 for LAAO device implantations. The predominant device used in LAAO procedures in our dataset was the first-generation Watchman device since the Watchman FLX and Amulet devices were not approved by the Food and Drug Administration (FDA) during the time frame of our study. The NIS is made possible by a Federal-State-Industry partnership sponsored by the Agency for Healthcare Research and Quality (AHRQ). The NIS is the largest all-payer inpatient healthcare database and is derived from non-Federal hospitals in all States and can be used for computing national estimates of healthcare utilization, costs, and outcomes [13]. The NIS provides discharge weights that are used for estimation of disease and procedure trends nationally. Due to the de-identified nature of NIS dataset, the need for informed consent and Institutional Review Board approval is waived.
2.2 Study population
Watchman device implantations were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes of 37.90 and 02L73DK, respectively, from our dataset. These codes have been extensively utilized in earlier studies for stratification of LAAO devices from administrative datasets [14,15,16]. Patients younger than 18 years and those with missing demographic data were excluded. Patients were stratified on the basis of age into two sub-groups, patients ≤ 80 years old and patients who were > 80 years of age. Baseline characteristics, procedural complications and inpatient outcomes including mortality (reported as a distinct categorical variable in the dataset), length of stay, and hospitalization costs were compared in Watchman recipients based on age sub-groups. We also analyzed the independent association of age (patients > 80 years versus patients ≤ 80 years) with outcomes of mortality, major complications (defined as composite of pericardial effusion requiring intervention, cardiac arrest, ischemic stroke/transient ischemic attack, hemorrhagic stroke, systemic embolism, myocardial infarction, and peripheral vascular complications which included AV fistula, pseudoaneurysm, access site hematoma, retroperitoneal bleeding, and venous thromboembolism), prolonged hospital stay (defined as length of stay > 1 day), and increased hospitalization cost (median hospitalization cost > 24,327$). For computing hospitalization costs, the cost-to-charge ratio files supplied by Healthcare Cost and Utilization Project were applied to the total hospital charges and adjusted for inflation to December 2018.
2.3 Statistical analysis
Descriptive statistics are presented as frequencies with percentages for categorical variables and as median with interquartile range (IQR) for continuous variables. Baseline characteristics were compared using a Pearson χ2 test and Fisher’s exact test for categorical variables and the Kruskal–Wallis H test for continuous variables. For crude comparison of procedural complications and in-hospital outcomes among the age groups, the Pearson χ2 test was utilized. For assessment of the independent association of age with outcomes including mortality, major complications, length of stay > 1 day, and median hospitalization cost > 24,327$, a single-step multivariable logistic regression model was utilized. Sex, race/ethnicity, CHA2DS2-VASc score, hospital size, and 29 Elixhauser co-morbidities (heart failure, valvular disease, pulmonary circulation disease, peripheral vascular disease, paralysis, neurological disorders, chronic pulmonary disease, diabetes without complications, diabetes with chronic complications, hypothyroidism, hypertension, renal failure, liver disease, peptic ulcer, acquired immune deficiency syndrome, lymphoma, metastatic cancer, solid tumor without metastasis, collagen vascular disease, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, chronic blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, psychoses, and depression) were used for adjustment of potential confounders. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using Statistical Package for Social Science (SPSS) version 26 (IBM Corp) and R version 3.6. Because of the complex survey design of NIS, sample weights, strata, and clusters were applied to raw data to generate national estimates.
3 Results
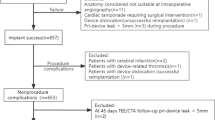
We extracted a total of 36,139 LAAO procedures for years 2015 to 2018. Of these, 74 procedures were excluded due to missing demographic patient data, thus yielding a final sample size of 36,065. Baseline characteristics of the study population stratified based on age are shown in Table 1. Out of 36,065 procedures, approximately 12,475 (34.6%) Watchman devices were implanted in patients aged greater than 80 years and 23,590 (65.4%) LAAO Watchman implantations occurred in patients who were aged 80 years or less. Elderly Watchman recipients (patients > 80 years) were more likely to be female, and had a higher burden of specific co-morbidities when compared to a relatively younger cohort of Watchman LAAO recipients (patients ≤ 80 years), including congestive heart failure (35.1% vs. 32.7%, p < 0.01), renal failure (25.1% vs. 23.4%, p < 0.01), and peripheral vascular disorders (10.9% vs. 9.3%, p < 0.01). The prevalence of coronary artery disease (7.7% vs. 7.7%, p = 1) and hypertension (85.3% vs. 86%, p = 0.06) was similar in both groups.
Table 2 shows crude in-hospital complications after the implantation of Watchman LAAO devices in our cohort. The prevalence of major complications was higher in elderly AF patients undergoing Watchman implantation when compared to a relatively younger cohort (6.7% vs. 5.7%, p < 0.01). This was primarily driven by an increased prevalence of any cardiovascular complication (3.8% vs. 2.8%, p < 0.01) and any neurological complication (1.1% vs. 0.7%, p < 0.01) in the elderly Watchman cohort. The crude prevalence of additional in-hospital outcomes in AF patients after LAAO Watchman implantation stratified by age subgroups is shown in Table 3. Elderly AF patients undergoing Watchman LAAO implantation experienced in-hospital mortality more frequently when compared to the younger patients (0.4% vs. 0.1%, p < 0.01). The prevalence of non-home discharges was also higher in elderly patients when compared to younger patients (10.7% vs. 7.4%, p < 0.01).
To analyze the independent association of age with important outcomes of mortality, major complications, prolonged length of stay (defined as length of stay > 1 day), and hospitalization costs (defined as median cost > 24,327$), a single-step multivariate logistic regression model was created to adjust for potential confounding variables and is shown in Fig. 1. After multivariable adjustment, age > 80 years was found to be an independent predictor of inpatient mortality after Watchman LAAO implantation (adjusted odds ratio [aOR] 4.439, 95% confidence interval [CI] 2.391–8.239). However, after multivariable adjustment, age > 80 years was not associated with major complications (aOR 1.084, 95% CI 0.971–1.211), prolonged length of stay (aOR 0.943, 95% CI 0.88–1.101), or increased hospitalization costs (aOR 0.909, 95% CI 0.865–0.955).
4 Discussion
In this large and nationally representative study of AF patients undergoing Watchman LAAO implantation stratified based on age, we report several important findings: (1) Approximately 35% of Watchman LAAO implantations occurred in AF patients > 80 years of age. (2) As expected, elderly patients had a higher burden of some co-morbidities such as congestive heart failure, renal failure, and peripheral vascular disease. (3) The crude prevalence of major complications was higher in elderly AF patients undergoing Watchman LAAO implantation and largely driven by cardiovascular and neurological complications. (4) After multivariable adjustment for potential confounders, age > 80 years was a predictor of inpatient mortality in AF patients undergoing LAAO using a Watchman device but was not associated with other outcomes of major complications, prolonged length of stay, and increased hospitalization costs. These data suggest that although a more frail and comorbid population, elderly AF patients undergoing LAAO implantation appear to have similar risk of adverse events (except in-hospital death and for which the absolute crude difference was small among the two groups, 0.4% vs. 0.1%) when accounting for other comorbidities that are often present with advanced age.
LAAO using an earlier generation Watchman device has shown to be non-inferior to warfarin in the landmark PROTECT AF (Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial) and PREVAIL (Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy) trials [7, 8]. Although no distinct analysis was done for elderly patients in the PROTECT AF and PREVAIL trials, the proportion of patients aged > 75 years was 41% and 52%, respectively, in these trials. Since the comparator arm of these trials constituted subjects tolerating warfarin, advanced age patients (> 80 years) were not well represented in previous randomized controlled trials due to either a prior bleeding event or an increased bleeding risk from underlying comorbidities that are often associated with aging. More recently, newer generation Watchman FLX and Amplatzer Amulet devices are approved by Food and Drug Administration and were studied in the PINNACLE FLX (Primary Outcome Evaluation of a Next Generation Left Atrial Appendage Closure Device) and Amulet IDE (Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial) trials [17, 18]. The reported prevalence of patients > 75 years of age was 50.5% in the PINNACLE FLX trial. In an age-stratified analysis from the Amulet IDE trail, no differences in the primary efficacy and safety endpoints were noted in patients < 70 years and ≥ 70 years after implantation of Amplatzer Amulet or the Watchman device. Our national cohort of Watchman LAAO recipients demonstrated that approximately 35% of such implantations occurred in elderly AF patients who were more than 80 years, and this patient group was not well represented in the aforementioned trials. The plausible explanation for such higher utilization rate of Watchman LAAO may be that elderly patients > 80 years are more prone to significant bleeding events while on OACs [5, 6] and LAAO provides a viable alternative to such patients for reduction of stroke risk with simultaneous attenuated risk of significant bleeding. Furthermore, our data support the fact that implanting physicians should not hesitate in offering LAAO to eligible elderly patients as it affirmed that these patients do not have an inherent increased risk of major adverse events after LAAO implantation.
Few earlier observational studies have assessed the association of age with procedural related outcomes after LAAO device implantation. In a study of more than 1000 patients from the AMPLATZER cardiac plug multicenter registry, Friexa et al. demonstrated that procedural success was similar at more than 97% in AF patients who were above 75 years of age as compared to the younger cohort after the implantation of the AMPLATZER LAAO device [10]. They also showed no difference in the rate of procedure-related adverse events in their cohort of older and younger AMPLATZER recipients (5.1% vs. 3.2%, p = 0.17). In another study of more than 1000 patients undergoing LAAO using a Watchman device, Gonzalez et al. assessed outcomes in elderly patients who were ≥ 85 years old and compared them with a younger cohort of patients [11]. They also found similar procedural success of Watchman implantation in both age groups (98.8% vs. 98.5%, p = 0.99). The risk of significant procedure-related adverse events was also similar in both age groups in their cohort of patients undergoing Watchman implantation (2.6% vs. 3.1%, p = 0.80). In a more recent study of 6779 LAAO procedures, Sanjoy et al. [12] demonstrated that older patients (≥ 80 years old) experienced a higher prevalence of major adverse events when compared to a younger cohort of LAAO recipients (6% vs. 4.6%, p = 0.01). This increased rate of major adverse events in their older cohort was primarily driven by excess magnitude of mortality and cardiovascular complications. Our study expands upon the previous studies, as it is the largest study that adjusted for several comorbid conditions, and also demonstrated increased adjusted risk of mortality in older LAAO device recipients and further depicted that the adjusted risk of other important outcomes such as major complications, prolonged length of stay, and increased hospitalization cost was not different in both the studied age groups. These findings have important implications in the stratification of such patients and can guide implanting physicians with respect to risk/benefit discussion of LAAO in elderly AF patients. Specifically, although elderly patients undergoing LAAO frequently have comorbid conditions, when statistical adjustment for these conditions is performed, the risk of adverse events in this population is not severely elevated except for the risk of mortality.
5 Limitations
The results of our current study should be interpreted in the context of following limitations. First, the NIS relies on ICD codes for disease and procedure identification which may be subjected to errors. It should be noted, however, that the NIS has a robust quality control program that minimizes miscoding and ensures data integrity [13]. Second, long-term outcomes cannot be ascertained from the present dataset as NIS does not follow patients longitudinally. Additionally, it is not possible to discern the cause of inpatient mortality from the NIS. Third, NIS does not provide information on anti-coagulation strategy utilized in AF patients and also does not capture any data on frailty which may be sources for potential confounders or reverse causality in our multivariate analysis. Fourth, the NIS only caters to inpatient admissions and does not provide information on outpatient encounters. However, it should be noted that inpatient admission is required for reimbursement of a LAAO device [19]; and hence, our study constitutes a well representative national sample of Watchman implantations in the US in the contemporary period. Fifth, the LAAO device implantations analyzed in this study were first-generation Watchman as newer LAAO devices such as the Watchman FLX and Amulet were not approved by the FDA during our study time period. Additionally, no data on hospital or the operator procedural volume is provided by the NIS.
6 Conclusion
Our study showed that a significant proportion of LAAO device implantations (approximately 35%) occurred in elderly AF patients. The crude prevalence of major complications and mortality was higher in elderly AF patients undergoing LAAO device implantation. After multivariate adjustment modeling, age > 80 years was associated with inpatient mortality in our national cohort of LAAO device recipients, but not with other adverse events. Advanced age should not prevent the implanting physicians against offering LAAO device implantation for eligible patients.
Data availability
The data that support the finding of this study are available from the first author (MBM) upon reasonable request.
References
Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6.
Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–61.
Saposnik G, Gladstone D, Raptis R, Hart RG. Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44:99–104.
Seet RC, Zhang Y, Wijdicks EF, Rabinstein AA. Relationship between chronic atrial fibrillation and worse outcomes in stroke patients after intravenous thrombolysis. Arch Neurol. 2011;68:1454–8.
Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol. 2007;6:981–93.
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67.
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–42
Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12.
Munir MB, Khan MZ, Darden D, Pasupula DK, Balla S, Han FT, Reeves R, Hsu JC. Contemporary procedural trends of Watchman percutaneous left atrial appendage occlusion in the United States. J Cardiovasc Electrophysiol. 2021;32:83–92.
Freixa X, Gafoor S, Regueiro A, Cruz-Gonzalez I, Shakir S, Omran H, Berti S, Santoro G, Kefer J, Landmesser U, Nielsen-Kudsk JE, Sievert H, Kanagaratnam P, Nietlispach F, Gloekler S, Aminian A, Danna P, Rezzaghi M, Stock F, Stolcova M, Costa M, Ibrahim R, Schillinger W, Park JW, Meier B, Tzikas A. Comparison of efficacy and safety of left atrial appendage occlusion in patients aged <75 to ≥ 75 years. Am J Cardiol. 2016;117:84–90.
Cruz-González I, Ince H, Kische S, Schmitz T, Schmidt B, Gori T, Foley D, de Potter T, Tschishow W, Vireca E, Stein K, Boersma LV. Left atrial appendage occlusion in patients older than 85 years. Safety and efficacy in the EWOLUTION registry. Rev Esp Cardiol (Engl Ed). 2020;73:21–27
Sanjoy SS, Choi YH, Sparrow RT, Jneid H, Dawn Abbott J, Nombela-Franco L, Azzalini L, Holmes DR, Alraies MC, Elgendy IY, Baranchuk A, Mamas MA, Bagur R. Outcomes of elderly patients undergoing left atrial appendage closure. J Am Heart Assoc. 2021;1: e021973.
Agency for Healthcare Research and Quality. Overview of the national inpatient sample (NIS). Rockville: AHRQ. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed on November 26, 2021
Khan MZ, Munir MB, Darden D, Pasupula DK, Balla S, Han FT, Reeves R, Hsu JC. Racial disparities in in-hospital adverse events among patients with atrial fibrillation implanted with a watchman left atrial appendage occlusion device: a US national perspective. Circ Arrhythm Electrophysiol. 2021;14: e009691.
Munir MB, Khan MZ, Darden D, Pasupula DK, Balla S, Han FT, Reeves R, Hsu JC. Pericardial effusion requiring intervention in patients undergoing percutaneous left atrial appendage occlusion: prevalence, predictors, and associated in-hospital adverse events from 17,700 procedures in the United States. Heart Rhythm. 2021;18:1508–15.
Munir MB, Khan MZ, Darden D, Nishimura M, Vanam S, Pasupula DK, Asad ZUA, Bhagat A, Zahid S, Osman M, Balla S, Han FT, Reeves R, Hsu JC. Association of chronic kidney disease and end-stage renal disease with procedural complications and in-hospital outcomes from left atrial appendage occlusion device implantation in patients with atrial fibrillation: insights from the national inpatient sample of 36,065 procedures. Heart Rhythm. 2021;O2(2):472–9.
Kar S, Doshi SK, Sadhu A, Horton R, Osorio J, Ellis C, Stone J Jr, Shah M, Dukkipati SR, Adler S, Nair DG, Kim J, Wazni O, Price MJ, Asch FM, Holmes DR Jr, Shipley RD, Gordon NT, Allocco DJ, Reddy VY; PINNACLE FLX Investigators. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX Trial. Circulation. 2021;143:1754–1762
Lakkireddy D, Thaler D, Ellis CR, Swarup V, Sondergaard L, Carroll J, Gold MR, Hermiller J, Diener HC, Schmidt B, MacDonald L, Mansour M, Maini B, O’Brien L, Windecker S. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): a randomized, controlled trial. Circulation. 2021;144:1543–52.
Centers for Medicare and Medicaid Services Coverage with Evidence Development (Left Atrial Appendage Occlusion). https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/LAAC. Accessed on November 26, 2021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Hsu reports receiving honoraria from Medtronic, Abbott, Boston Scientific, Biotronik, Janssen Pharmaceuticals, Bristol-Myers Squibb, Pfizer, Sanofi, Hillrom, Baylis Medical, Acutus Medical, iRhythm, Zoll Medical, and Biosense-Webster, research grants from Biotronik and Biosense-Webster, and has equity interest in Vektor Medical. All the other authors have no competing interests to disclose.
Ethical approval and informed consent
The need for these entities was waived due to the deidentified nature of National Inpatient Sample database and its public availability.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Munir, M.B., Khan, M.Z., Darden, D. et al. Association of advanced age with procedural complications and in-hospital outcomes from left atrial appendage occlusion device implantation in patients with atrial fibrillation: insights from the National Inpatient Sample of 36,065 procedures. J Interv Card Electrophysiol 65, 219–226 (2022). https://doi.org/10.1007/s10840-022-01266-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01266-1