Abstract
Purpose
Integration of a 3D reconstruction of the left atrium into cardiac mapping systems can aid catheter ablation of atrial fibrillation (AF). The two most widely used systems are NavX Fusion and Cartomerge. We aimed to compare the clinical efficacy of these systems in a randomised trial.
Methods
Patients undergoing their first ablation were randomised to mapping using either NavX fusion or CartoMerge. Pulmonary vein isolation by wide area circumferential ablation was performed for paroxysmal AF with additional linear and fractionated potential ablation for persistent AF. Seven-day Holter monitoring was used for confirmation of sinus rhythm maintenance at 6 months.
Results
Ninety-seven patients were randomised and underwent a procedure. There was no difference in the primary endpoint of freedom from arrhythmia at 6 months (51% in the Cartomerge group vs. 48% in the NavX Fusion group, p = 0.76). 3D image registration was faster with Cartomerge (24 vs. 33 min, p = 0.0001), used less fluoroscopic screening (11 vs. 15 min, p = 0.039) with a lower fluoroscopic dose (840 vs. 1,415 mGyCm2, p = 0.043). There was a strong trend to lower ablation times in the Cartomerge group, overall RF time (3,292 s vs. 4,041, p = 0.07). Distance from 3D lesion to 3D image shell was smaller in the Cartomerge group (2.7 ± 1.9 vs. 3.3 ± 3.7 mm, p < 0.001).
Conclusions
Cartomerge appears to be faster and uses less fluoroscopy to achieve registration than NavX Fusion, but overall procedural times and clinical outcomes are similar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Catheter ablation procedures for atrial fibrillation (AF) are performed with increasing frequency since the technique was first described over 15 years ago [1]. Technological developments appear to have reduced X-ray doses and may help newer centres establish an AF ablation programme [2].
3D mapping systems are widely used in catheter ablation of AF. Integration of a previously acquired image of the left atrium (LA) into the electroanatomical map offers several potential advantages, including visualization of the complex anatomy of the left atrium, reduction of fluoroscopy time and improved results [3–8]. The two most widely used systems are Cartomerge (Biosense Webster, Diamond Bar, CA, USA) and NavX Fusion (St. Jude Medical Inc., Saint Paul, MN, USA). Although both systems have been independently validated [9–12], their clinical utility has not previously been directly compared in a randomised trial.
2 Methods
2.1 Study population
All eligible patients with either paroxysmal or persistent AF undergoing their first catheter ablation at St Bartholomew’s Hospital were invited to participate in this study. All patients had symptomatic documented AF and had failed 1 or more class I and/or class III antiarrhythmic drugs. Exclusion criteria were <18 years of age, left ventricular ejection fraction <40%, known congenital heart disease or involvement in another study. The study was approved by the Outer North East London Research and Ethics committee (study number 07/4Q0603/66), and patients gave written informed consent. The design of the study followed the Consort guidelines for randomised trials [13].
2.2 LA image acquisition
A multislice helical contrast CT was performed using GE Light-speed Ultra 8-slice scanner (GE Healthcare Technologies, Waukesha, WI, USA). The technique used for CT acquisition has been previously described [4]. Towards the end of the recruitment period, it became possible to use a cardiovascular magnetic resonance (CMR) scan image for segmentation of the LA, using a Seimens Magnotron 1.5-T MRI with cardiac gating.
2.3 Operator experience
Our centre had experience of >100 cases using image integration for each system at the start of patient recruitment. Image integration was performed by operators (ME, MD, SS, RS) assisted by technicians, each of whom had performed >20 image integrations and undergone training prior to the trial.
2.4 Procedure
The procedure was performed in the post-absorptive state under conscious sedation. In patients with a CHADS2 score of >1, oral anticoagulation was administered for at least 4 weeks prior to the procedure. Transesophageal echocardiography was performed within 24 h prior to the procedure to exclude LA thrombus.
Local anaesthesia and conscious sedation was used for all procedures. Venous access, number and size of sheaths were at the operator’s discretion. In the majority of cases, three venous sheaths (1 × 7 F short sheath, 2 × 8 F long sheath) were inserted via the right femoral vein without ultrasound guidance. If the patient was in AF at the start of the case, a decapolar catheter was positioned in the coronary sinus; otherwise, a quadripolar catheter was used. Two transseptal punctures were performed under fluoroscopic guidance with an Endry’s coaxial needle using continuous pressure monitoring. Two long Mullin’s sheaths were advanced into the LA, and a pulmonary vein mapping catheter (OrtbiterTM Woven, Bard Electrophysiology, Lowell, MA, USA) and an irrigated tip ablation catheter (either NavistarTM (Cartomerge group) or CelsiusTM (NavX Fusion group) Thermocool, Biosense Webster Inc., Diamond Bar, CA, USA) were advanced through the sheaths.
2.5 Image integration
2.5.1 NavX fusion
The CT left atrium (CTLA) was imported into the proprietary VerisimoTM CT segmentation software. Image segmentation was performed as previously described [10], ensuring that pulmonary veins were visible to second-order branch points and the left atrial appendage (LAA) was included in the scan. The rendered 3D image was imported into the NavX study. A geometry of the LA was collected from the PV mapping catheter, with particular attention given to collecting points at the PV ostia. Further geometry was collected from the mapping catheter. Field scaling was applied which refers to a computer algorithm that uses the known fixed separation of poles on the mapping catheter and the PV catheter to calibrate the non-linear electrical field data to real 3D space. Field scaling effectively reduces the apparent flattening and stretching of the LA geometry, making it easier to perform image integration [11]. To integrate the image, 15 to 30 fiducial points that correspond to landmarks clearly visible on both CTLA and geometry are chosen. These often include, but are not limited to, venous bifurcations, the LAA, the intra-atrial septum, the mitral ring as well as several points on the roof, posterior wall and floor of the LA. When fiducial selection is complete, fusion is attempted. The geometry is rescaled to fit the segmented image, using the fiducials to effectively allow ‘stretching’ of the geometry to match the exported scan image. Thereafter, catheters can be located on the scanned CT image shell, the matched geometry or both.
2.5.2 Cartomerge
Previously acquired scan images were imported into the proprietary Cartomerge image processing tool software. Segmentation was performed, ensuring that veins were visible to second-order branch points, and the segmented image imported into the Cartomerge study. The ablation catheter was placed on the roof of each vein in turn and retrograde pulmonary venography performed to confirm its exact position in relation to venous branch points. The position of the ablation catheter was identified and a Carto landmark point taken. This point was linked to its corresponding location on the segmented CT/CMR shell. Once points in all four veins had been acquired, landmark registration was applied. According to operator preference, further surface registration could be applied. Image integration was considered successful if the operator considered it to give an accurate representation of catheter location within the atrial chamber.
2.6 Ablation
PV WACA was performed using an irrigated tip catheter with power limited to 30 W and temperature to 50°C, with the endpoint electrical isolation determined by a circular PV catheter. In patients in whom AF persisted, further ablation was performed using a combination of (a) roof line, (b) mitral isthmus line, (c) coronary sinus endocardial and epicardial (within the CS) lines and (d) complex fractionated electrograms. If at any stage AF organized into atrial tachycardia (AT), activation and entrainment mapping was performed. If AF persisted following the linear ablation and targeting of fractionated electrograms, DC cardioversion was performed. Cavotricuspid isthmus ablation was performed only if patients had previously documented typical atrial flutter or organized into this during other ablation.
2.7 Follow-up
All patients were reviewed in clinic at 3, 6 and 12 months following the procedure. If no episodes of AF recurrence had been documented, a 7-day Holter monitor was performed at 6 and 12 months.
2.8 Endpoints
The primary endpoint was freedom from AF at 6 months, with AF recurrence defined either by documentation of symptomatic arrhythmia or arrhythmia on a 7-day Holter monitor at 6-month follow-up. Secondary endpoints were procedural, screening and energy delivery times for all stages of the procedure and complications and freedom from AF at 1 year following a single procedure
A sub-study aimed to determine navigational accuracy of image registration in clinical use. Data were taken from a consecutive series of 21 patients in each group where image registration had been used throughout the case. The distance from LA lesions points to their corresponding nearest location on the integrated image shell was assessed. Techniques for this assessment in both technologies have been previously described [10, 14]. This assessment assumes that lesion points are placed on the endocardial surface of the LA; lesions placed outside the endocardial LA (e.g. within coronary sinus) were excluded in this analysis. In Cartomerge, the three-dimensional linear distance from each lesion to the nearest point on the integrated image shell is calculated within the Cartomerge software. An equivalent distance can be calculated from data exported from NavX fusion. Lesion coordinates are exported separately, initially as 3D lesions and subsequently as projected onto the nearest corresponding point of the integrated image (‘Project on Dif’). The primary objective of this sub-study was to compare observed accuracy during clinical use; therefore, both field scaling and surface registration were permitted. Offline analysis was performed using custom software to calculate linear distances between projected and 3D points for each lesion (Matlab, The Mathworks Inc., Natick, MA, USA).
2.9 Statistical analysis
Assuming an overall single procedure success rate at 6 months of 60%, 63 patients were required in each group to achieve an 80% chance of detecting a 10% difference (alpha = 0.05, two-tailed) in outcomes between groups. Univariate continuous variables were compared with Student’s T test or the Mann–Whitney U test as appropriate. Proportions were compared using the chi-squared test. Univariate survival analyses were performed by the Kaplan–Meier method, and multivariate survival regression analyses were performed using the Cox regression method. Statistical analyses were carried out using PASW 18.0 (SPSS Inc., Chicago, IL, USA) software and Graphpad Prism 4 (Graphpad Inc., San Diego, CA, USA). Analyses performed included data from all patients who underwent an ablation procedure. Data are presented on an intention-to-treat basis; hence, presented procedural data include information from patients who had unsuccessful image integration. A 6-week blanking period was observed, but timing of recurrence was ascribed to the earliest documented recurrence (even within this 6-week period). Values are given as means ± standard deviation unless otherwise stated.
3 Results
3.1 Randomisation and baseline factors
One hundred one patients were enrolled. The trial design and randomisation [13] is summarised in the Electronic Supplementary Material, Figure 1. A total of 97 patients underwent catheter ablation, and 98% of these received their assigned treatment. Two patients who were allocated NavX Fusion in randomisation underwent ablation with a non-trial mapping system due to computer malfunction of the patient interface unit. Four patients (three randomised to Cartomerge, one to NavX Fusion) did not complete a catheter ablation study following randomisation, all citing recent improvements in symptoms.
Baseline clinical factors did not differ between the two groups (Table 1). Twenty-five patients in the NavX Fusion group and 23 patients in the Cartomerge group were in persistent or longstanding persistent AF, with a median time since first episode of AF 59 months (range 2–240) vs. 63 months (8–306) (p = 0.66). An interim analysis of the primary endpoint following recruitment of 90 patients determined that statistical significance in the primary endpoint was unlikely to be reached, and recruitment was terminated at 101 patients.
3.2 Primary endpoint
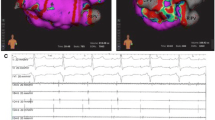
Catheter ablation was performed in 97 of 101 patients. The primary endpoint of freedom from AF off antiarrhythmic drugs at 6 months was reached by 24 patients in both groups (Cartomerge 51%, NavX fusion 48%, χ 2, p = 0.76). There was no difference in the time to recurrence of arrhythmia as measured by the log-rank statistic (p = 0.74) (Fig. 1).
Procedural outcomes are summarised in Table 2. At the end of the ablation procedure, 45 (96%) patients completing in the Cartomerge group and 50 patients (100%) in the NavX fusion group were in sinus rhythm (p = 0.58). Two patients from the Cartomerge group remained in an arrhythmia at the end of the procedure despite attempts at DC cardioversion, one in AF, the other in a left-sided AT. In patients with persistent AF, DC cardioversion was used to achieve sinus rhythm in 17 (74%) patients in the Cartomerge group and 15 (60%) patients in the NavX fusion group (χ 2, p = 0.12). After 6 months clinical follow-up, 66% patients in the Cartomerge group and 68% in the NavX fusion group were in sinus rhythm following a single procedure (p = 0.91) as documented with a 12 lead ECG in clinic. At 1 year, 44% of patients in the Cartomerge group vs. 38% patients in NavX Fusion group remained free from any documented arrhythmia on 7-day Holter and off antiarrhythmic drugs following a single procedure (p = 0.6).
3.3 Procedural factors
Procedural time points are summarised in Table 3 and Fig. 2. Time taken for overall ablation procedures was similar between Cartomerge and NavX fusion, but procedures for persistent atrial fibrillation (persAF) were significantly longer than those for paroxysmal atrial fibrillation (median [range]) (199 [74–360] vs. 294 [90–520] min, p < 0.0001). Registration was faster with Cartomerge (24 vs. 33 min, p = 0.0001), used less fluoroscopic screening (11 vs. 15 min, p = 0.039) and had a lower fluoroscopic dose (840 vs. 1,415 mGyCm2, p = 0.043) than with NavX Fusion. There was a trend to lower ablation times in the Cartomerge group (overall RF time 3,292 s vs. 4,041, p = 0.07). All pulmonary veins were confirmed to be isolated at the end of the case in 44 patients (94%) in the Cartomerge group vs. 47 patients (94%) (p > 0.9). The time taken for initial isolation of each pair of PVs was similar between groups. Twenty-four patients (51%) in the Cartomerge group vs. 23 patients (46%) had pulmonary electrical reconnection apparent during the case requiring further ablation. Times from procedure start (local anaesthetic to skin) to completion of isolation of both sets of veins were not significantly different between groups (164 vs. 177 min, p = 0.22).
Key procedural time points, shown as cumulatives (rather than true order in which ablations progressed). This illustrates the contributions of each procedural stage to the overall time taken. Inset numerals show times for each stage as mean ± SD. Time from start of case to registration was significantly shorter with Cartomerge, but this did not translate into overall reduced case time. pAF paroxysmal atrial fibrillation, persAF persistent atrial fibrillation, DTSP double transseptal puncture, RPV right pulmonary vein, LPV left pulmonary vein
3.4 Image integration
3D image integration (merge or fusion) was completed and used more often with Cartomerge than NavX fusion (44 patients (94%) vs. 39 patients (78%); p = 0.046). The poor quality of CT images following segmentation was given as the reason for failure of image integration in all three patients in the Cartomerge group. Reasons for failure of NavX Fusion image integration were listed as failure to achieve adequate fusion (five patients), inability to import CT images (file format mismatch) (one patient), instability of the reference electrode (two patients) and poor quality CT images (three patients). CT integration was abandoned during the case following achieving adequate registration in a further three patients in each group. In all cases, this was because of apparent evolving mismatch of anatomy with registration. 3D registration was successful for the entire case for 87% of patients in the Cartomerge group and 72% of patients in the NavX fusion group (p = 0.12).
3.5 Navigational accuracy of image integration
Three thousand one hundred seventy-four LA lesions from Cartomerge and 2,712 lesions from NavX fusion were analysed (from 21 patients in each group with subjectively satisfactory image integration). Cartomerge lesions were significantly closer to the 3D shell than those of NavX fusion (2.0 ± 0.5 vs. 3.4 ± 0.9 mm, p < 0.0001). The maximum distance from lesion to shell per patient was lower in Cartomerge than with NavX fusion (10.2 ± 0.7 vs. 15.9 ± 1.1 mm, p = 0.0002; Table 4).
3.6 Cost comparison
The cost per case of the comparative navigation systems was calculated using methods as previously described [8], where using a quadripolar catheter as a reference cost unit of 1 (Table 5). There was no difference between the actual equipment costs of using either system (30.1 ± 0.2 vs. 29.9 ± 0.2, p = 0.5). This figure does not take account of the ability to re-use Carto location patches between consecutive patients within a 24-h period, which can substantially reduce costs if more than one patient is undergoing CA of AF in the same catheter laboratory on that day.
3.7 Adverse events
A total of 11 adverse events were reported during the study (11%). There were three cases of cardiac tamponade, one (2%) in the Cartomerge group and two (4%) in NavX fusion group. All were successfully treated with pericardiocentesis. Six patients, four (9%) in the Cartomerge group and two (4%) in the NavX fusion group, developed haematomas requiring hospital admission to be extended. Two patients (4%) in the Cartomerge group developed minor focal neurological symptoms following the procedure, a cerebrovascular event was confirmed on MRI in one patient, and imaging was normal in the other patient. Both patients made a full functional recovery within 48 h. No symptomatic pulmonary venous stenosis was reported in either group, and no asymptomatic pulmonary venous stenosis was observed in any case undergoing a redo procedure or on further imaging performed for clinical indications.
4 Discussion
The principal findings of this study are that Cartomerge allowed a slightly faster and more accurate registration of the reconstructed LA than NavX fusion, but overall procedural times and clinical outcomes were unaffected by mapping system used. A reduction in X-ray screening associated with registration using Cartomerge was observed, but this was small in comparison to overall screening and did not translate to an overall reduction in X-ray use. Less ablation time was used with Cartomerge than with NavX fusion, and similar procedural results were achieved.
Clinical results, measured as freedom from AF at 6 months, were not different between groups. Although one system cannot be claimed to be superior overall to another for the catheter ablation of atrial fibrillation, the slightly faster procedural stages seen with Cartomerge and greater accuracy, particularly in image registration, may encourage its use for pulmonary vein isolation procedures.
This is the first prospective, randomised clinical trial comparing the two most widely commercially available 3D mapping systems. Image integration of a previously acquired anatomical dataset to 3D mapping systems offers several advantages in the ablation of complex arrhythmias, particularly AF where a good knowledge of anatomy is key to procedural success. The technique of image integration, particularly the process of registration, with Cartomerge and NavX fusion is quite distinct.
The Carto XP system locates catheter position in 3D space by trigonometry from three separate magnetic (linear) fields. The relative field strength in three planes enables location of a proprietary mapping catheter. User-selected locations (‘points’) can link 3D locations with anatomical boundaries, and known anatomical landmarks can be used for rapid registration of a previously LA image. The technique used in this trial has previously been described [4]. As magnetic fields vary in a linear manner (actually log-linear) with distance, and a fixed movement of the ablation catheter tip produces a corresponding and predictable change in magnetic fields detected by the catheter tip. Anatomical data can thus be imported into the Carto system without need for further calibration.
In contrast, NavX uses three orthogonal high-frequency electric fields to locate catheter position. Impedance changes due to catheters movement can be tracked in three dimensions and relative positions of catheters derived. Unlike magnetic fields, electrical fields within the human body are non-linear; thus, a fixed deviation of catheter produces an impedance change dependent on the exact location of the catheter within the body. The non-linearity of the NavX maps often gives an apparent anteroposterior flattening of the LA, and the anatomic dataset cannot be simply matched to position, as with Carto [10]. Field scaling and image fusion attempt to overcome this non-linearity. Previous work by our group has confirmed that good anatomical accuracy can be obtained by NavX fusion when field scaling is performed and an adequate number of fiducial points selected. The presented data showing a navigational accuracy in clinical use of NavX Fusion of 3.4 ± 0.9 mm is in extremely close agreement with the accuracy of 3.4 ± 1.6 mm observed by Brooks and colleagues [11] in a validation study.
Our results imply that image integration with NavX fusion is slower than Cartomerge. More ablation appeared to be required to achieve a similar clinical result with NavX fusion, possibly reflecting the higher accuracy of image registration with Cartomerge. These data give a mechanistic insight into improvements in outcomes associated with image integration with Carto XP seen in registry data, improvements not replicated with NavX fusion [15].
Contributors to the slower registration process of NavX fusion were not formally examined in this study. NavX fusion requires the creation of a full LA NavX geometry to allow image registration, whilst Cartomerge allows image registration after acquisition of as few as three points; further surface registration points can be targeted subjectively thus minimising number of acquired points. We hypothesise that the conceptually simpler Cartomerge image registration thus has fewer steps and is less time-consuming. A recent retrospective analysis of Carto- vs. NavX-guided ablation (without specifying CT integration) also found that Carto-guided procedures were more rapid overall than those using NavX [16]. Thus, time differences may not be entirely attributable to differences in CT integration technique.
Our findings are important when considering the future of 3D mapping technology in catheter ablation. Technological advances have recently allowed systems to integrate positional data from both impedance and magnetic data, aspiring to overcome limitations of both systems. Our data suggest that image integration in new systems would best be primarily based on magnetic location data. We believe that the utility, stability and accuracy of image integration in these new systems should be independently validated in the clinical setting.
We observed no differences in complication rates between groups. Neither symptomatic nor asymptomatic pulmonary vein stenosis was observed in this study, a purported benefit of 3D image integration [7].
5 Limitations of this study
Since this study, a number of updates to both the technologies studies have been developed. The results of this study should be extrapolated to these newer system versions with caution, but these newer versions are likely to be available and used in a limited number of centres for some time, and our data will therefore remain relevant. Our study was performed with operators experienced in both systems; a comparison of the learning curve using either system was not undertaken.
In comparing the two mapping systems, to obtain optimal outcomes and to represent best practice in both groups, image integration was used. However, the complex geometries created by NavX system are often adequate to perform safe catheter ablation without 3D image integration. CartoXP anatomical maps appear simple and schematic in comparison. Image integration may thus hold a greater potential benefit for the Carto system over NavX. Indeed, when using NavX in clinical practice, many physicians use a pre-procedure CT to identify and exclude variant pulmonary venous anatomy; thereafter, the geometry is used alone during ablation. Our clinical practice has moved away from routine image integration with NavX fusion as a direct result of the presented data.
6 Conclusions
Cartomerge offers faster and more reliable image registration than NavX fusion and may require less X-ray screening. Absolute differences between systems are small and do not translate into important differences in procedural length, cost or clinical outcome. Both systems can be considered equivalent for the first time of catheter ablation of atrial fibrillation.
References
Cappato, R., Calkins, H., Chen, S., Davies, W., Iesaka, Y., Kalman, J., et al. (2005). Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation, 111(9), 1100–1105.
Cappato, R., Calkins, H., Chen, S., Davies, W., Iesaka, Y., Kalman, J., et al. (2010). Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. Arrhythmia and Electrophysiology, 3(1), 32–38.
Powell, B., & Packer, D. (2009). Does image integration improve atrial fibrillation ablation outcomes, or are other aspects of the ablation the key to success? Europace, 11(8), 973–974.
Kistler, P., Rajappan, K., Harris, S., Earley, M., Richmond, L., Sporton, S., et al. (2008). The impact of image integration on catheter ablation of atrial fibrillation using electroanatomic mapping: a prospective randomized study. European Heart Journal, 29(24), 3029–3036.
Della Bella, P., Fassini, G., Cireddu, M., Riva, S., Carbucicchio, C., Giraldi, F., et al. (2009). Image integration-guided catheter ablation of atrial fibrillation: a prospective randomized study. Journal of Cardiovascular Electrophysiology, 20(3), 258–265.
Bertaglia, E., Bella, P., Tondo, C., Proclemer, A., Bottoni, N., De Ponti, R., et al. (2009). Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMerge Italian Registry. Europace, 11(8), 1004–1010.
Bertaglia, E., Brandolino, G., Zoppo, F., Zerbo, F., & Pascotto, P. (2008). Integration of three-dimensional left atrial magnetic resonance images into a real-time electroanatomic mapping system: validation of a registration method. Pacing and Clinical Electrophysiology, 31(3), 273–282.
Earley, M., Showkathali, R., Alzetani, M., Kistler, P., Gupta, D., Abrams, D., et al. (2006). Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. European Heart Journal, 27(10), 1223–1229.
Tops, L., Bax, J., Zeppenfeld, K., Jongbloed, M., Lamb, H., van der Wall, E., et al. (2005). Fusion of multislice computed tomography imaging with three-dimensional electroanatomic mapping to guide radiofrequency catheter ablation procedures. Heart Rhythm, 2(10), 1076–1081.
Richmond, L., Rajappan, K., Voth, E., Rangavajhala, V., Earley, M., Thomas, G., et al. (2008). Validation of computed tomography image integration into the EnSite NavX mapping system to perform catheter ablation of atrial fibrillation. Journal of Cardiovascular Electrophysiology, 19(8), 821–827.
Brooks, A., Wilson, L., Kuklik, P., Stiles, M., John, B., Shashidhar, et al. (2008). Image integration using NavX Fusion: initial experience and validation. Heart Rhythm, 5(4), 526–535.
Dong, J., Dalal, D., Scherr, D., Cheema, A., Nazarian, S., Bilchick, K., et al. (2007). Impact of heart rhythm status on registration accuracy of the left atrium for catheter ablation of atrial fibrillation. Journal of Cardiovascular Electrophysiology, 18(12), 1269–1276.
Moher, D. S. K., & Altman, D. G. (2001). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet, 357(9263), 1191–1194.
Dong, J., Calkins, H., Solomon, S., Lai, S., Dalal, D., Lardo, A., et al. (2006). Integrated electroanatomic mapping with three-dimensional computed tomographic images for real-time guided ablations. Circulation, 113(2), 186–194.
Hunter, R., Ginks, M., Ang, R., Diab, I., Goromonzi, F., Page, S., et al. (2010). Impact of variant pulmonary vein anatomy and image integration on long-term outcome after catheter ablation for atrial fibrillation. Europace, 12(12), 1691–1697.
Khaykin, Y., Oosthuizen, R., Zarnett, L., Wulffhart, Z., Whaley, B., Hill, C., et al. (2011). CARTO-guided vs. NavX-guided pulmonary vein antrum isolation and pulmonary vein antrum isolation performed without 3-D mapping: effect of the 3-D mapping system on procedure duration and fluoroscopy time. Journal of Interventional Cardiac Electrophysiology, 30(3), 233–240.
Conflicts of interest
M.F. and V.B. are recipients of St Jude Research Fellowships. M.F. is the recipient of a Wellcome Trust Research Training Fellowship. St Jude Medical had no part in the design, implementation or reporting of this study. R.J.S. is a member of the scientific advisory board for Biosense Webster. He is listed on the Speakers Bureau for Endocardial Solutions and has received payment for lectures sponsored by them. All authors have received support for travel to international meetings from Guidant, Medtronic, St Jude Medical, Endocardial Solutions, and Biosense Webster.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Registered at ClinicalTrials.gov: Trial # NCT01432743
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 270 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Finlay, M.C., Hunter, R.J., Baker, V. et al. A randomised comparison of Cartomerge vs. NavX fusion in the catheter ablation of atrial fibrillation: The CAVERN Trial. J Interv Card Electrophysiol 33, 161–169 (2012). https://doi.org/10.1007/s10840-011-9632-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-011-9632-7