Abstract
Purpose
Patients with polycystic ovarian morphology (PCOM) make up 20% cases for assisted reproductive technology (ART). Folliculogenesis is impaired in PCOS. Signaling molecules are involved in follicle development. Dysregulations of intrafollicular environment and signaling molecules are observed in PCOS. Granulosa cells (GCs) and oocytes secrete molecules into follicular fluid by exocytosis of SNAREs. The aim of this study is to evaluate vesicle transport and vesicle fusion proteins (SNAREs) in GCs from PCOS patients who have undergone IVF treatment.
Methods
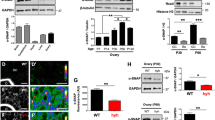
Follicular fluids were collected from patients who undergo IVF/ICSI with the diagnosis of male factor (n = 10) and PCOS (n = 10) patients. GCs were separated and cultured. Each group of GCs was stimulated with FSH-hCG. The cells were examined under electron microscope. Immunofluorescent labeling was performed on cells for Stx6, SNAP25, StxBP1, FSHr, and KITL. Integrated density was analyzed from images of Stx6, SNAP25, StxBP1, FSHr, and KITL.
Results
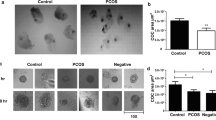
Intercellular communication occurs by signal molecules; Stx6, SNAP25, and StxBP1 fusion proteins involved in exocytosis were decreased in the GCs of PCOS. There was no increase in in vitro stimulation with FSH-hCG either. In the electron microscope, it was observed that exocytosis of the vesicles was disrupted.
Conclusions
Exocytosis and vesicular dynamics are among the basic physiological functions of human steroidogenic granulosa cells. Follicle development is necessary for production of competent oocytes and ovulation. Understanding the pathophysiology of PCOS at follicular level is important for disease management. According to our findings, deficits in vesicular dynamics of human granulosa cells in may be central to the treatment strategy for PCOS patients.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author, [SFM]. The data are not publicly available due to the privacy of research participants.
References
Fauser BCJM, Tarlatzis F, et al. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7. https://doi.org/10.1093/HUMREP/DEH098.
Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Prim. 2016;2:16057. https://doi.org/10.1038/nrdp.2016.57.
Waldman IN, Legro RS. Polycystic ovary syndrome. In: LEUNG PCK, ADASHI EY (eds) The Ovary: Third Edition, Third Edit. Elsevier; 2019. pp 415–435.
Torpy JM, Lynm C, Glass RM. Polycystic ovary syndrome. J Am Med Assoc. 2007;297:554. https://doi.org/10.1001/jama.297.5.554.
Andreeva P, Milachich T, Shterev A. ART treatment of infertile patient with PCO. In: Polycystic Ovarian Syndrome Conference. Endocrinol Metab Syndr. 2015;4(4):85 (Seattlei USA).
Webber LJ, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet (London, England). 2003;362:1017–21. https://doi.org/10.1016/s0140-6736(03)14410-8.
Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24:195–203.
Shimada M, Yanai Y, Okazaki T, et al. Synaptosomal-associated protein 25 gene expression ıs hormonally regulated during ovulation and ıs ınvolved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487–502. https://doi.org/10.1210/me.2007-0042.
Tuck AR, Robker RL, Norman RJ, et al. Expression and localisation of c-kit and KITL in the adult human ovary. J Ovarian Res. 2015;8:31. https://doi.org/10.1186/s13048-015-0159-x.
Wendler F, Tooze S. Syntaxin 6: The promiscuous behaviour of a SNARE protein. Traffic. 2001;2:606–11. https://doi.org/10.1034/j.1600-0854.2001.20903.x.
Pollard TD, Earnshaw WC, Lippincott-Schwartz J, Johnson GT. Secretory membrane system and Golgi apparatus. In: Cell Biology, Third Edit. Elsevier; 2017. pp 351–376.
Grosse J, Bulling A, Brucker C, et al. Synaptosome-associated protein of 25 kilodaltons in oocytes and steroid-producing cells of rat and human ovary: molecular analysis and regulation by gonadotropins1. Biol Reprod. 2000;63:643–50. https://doi.org/10.1095/biolreprod63.2.643.
Arcos A, de Paola M, Gianetti D, et al. α-SNAP is expressed in mouse ovarian granulosa cells and plays a key role in folliculogenesis and female fertility. Sci Rep. 2017;7:11765. https://doi.org/10.1038/s41598-017-12292-9.
Hong W. SNAREs and traffic. Biochim Biophys Acta - Mol Cell Res. 2005;1744:120–44. https://doi.org/10.1016/J.BBAMCR.2005.03.014.
Lin Y, Hou X, Shen W-J, et al. SNARE-mediated cholesterol movement to mitochondria supports steroidogenesis in rodent cells. Mol Endocrinol. 2016;30:234–47. https://doi.org/10.1210/me.2015-1281.
Kuliawat R, Kalinina E, Bock J, et al. Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic beta-cells. Mol Biol Cell. 2004;15:1690–701. https://doi.org/10.1091/MBC.E03-08-0554.
Perera HKI, Clarke M, Morris NJ, et al. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol Biol Cell. 2003;14:2946. https://doi.org/10.1091/MBC.E02-11-0722.
Jung JJ, Inamdar SM, Tiwari A, Choudhury A. Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6. Biosci Rep. 2012;32:383–91.
Reverter M, Rentero C, Garcia-Melero A, et al. Cholesterol regulates syntaxin 6 trafficking at trans-Golgi network endosomal boundaries. Cell Rep. 2014;7:883–97. https://doi.org/10.1016/j.celrep.2014.03.043.
Graham ME, Prescott GR, Johnson JR, et al. Structure-function study of mammalian Munc18–1 and C. elegans UNC-18 implicates domain 3b in the regulation of exocytosis. PLoS One. 2011;6. https://doi.org/10.1371/journal.pone.0017999.
STXBP1 - Syntaxin-binding protein 1 - Homo sapiens (Human) - STXBP1 gene & protein. https://www.uniprot.org/uniprot/P61764. Accessed 21 Feb 2021.
Zhang K, Wang T, Liu X, et al. CASK, APBA1, and STXBP1 collaborate during insulin secretion. Mol Cell Endocrinol. 2021;520:111076. https://doi.org/10.1016/j.mce.2020.111076.
Campbell JR, Martchenko A, Sweeney ME, et al. Essential role of syntaxin-binding protein-1 in the regulation of glucagon-like peptide-1 secretion. Endocrinol (United States). 2020;161. https://doi.org/10.1210/endocr/bqaa039.
İnfertilite ve Genetik Yönü. https://dijitalakademi.turkiyeklinikleri.com/flippage/tibbi-genetik-ozel/4-3/tr-index.html#p=20. Accessed 25 Apr 2021
Zırh S, Erol S, Zırh EB, et al. A new isolation and culture method for granulosa cells. Cell Tissue Bank. 2021. https://doi.org/10.1007/s10561-021-09929-5.
Costello MF, Misso ML, Balen A, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open. 2019;2019:1–24. https://doi.org/10.1093/hropen/hoy021.
Garcia-Velasco JA, Rodríguez S, Agudo D, et al. FSH receptor in vitro modulation by testosterone and hCG in human luteinized granulosa cells. Eur J Obstet Gynecol Reprod Biol. 2012;165:259–64. https://doi.org/10.1016/j.ejogrb.2012.08.020.
Szilágyi A, Bártfai G, Mánfai A, et al. Low-dose ovulation induction with urinary gonadotropins or recombinant follicle stimulating hormone in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2004;18:17–22. https://doi.org/10.1080/09513590310001651731.
Wu L, Fang Q, Wang M, et al. Effect of weight loss on pregnancy outcomes, neuronal-reproductive-metabolic hormones and gene expression profiles in granulosa cells in obese infertile PCOS patients undergoing IVF-ET. Front Endocrinol (Lausanne). 2022;13. https://doi.org/10.3389/FENDO.2022.954428.
Laven JSE. Follicle stimulating hormone receptor (FSHR) polymorphisms and polycystic ovary syndrome (PCOS). Front Endocrinol (Lausanne). 2019;10:23. https://doi.org/10.3389/FENDO.2019.00023.
Peak TC, Su Y, Chapple AG, et al. Syntaxin 6: A novel predictive and prognostic biomarker in papillary renal cell carcinoma. Sci Rep. 2019;9. https://doi.org/10.1038/s41598-019-39305-z.
Martín-Martín B, Nabokina SM, Blasi J, et al. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood. 2000;96:2574–83.
Kádková A, Radecke J, Sørensen JB. The SNAP-25 protein family. Neuroscience. 2018. https://doi.org/10.1016/J.NEUROSCIENCE.2018.09.020.
Wiser O, Trus M, Hernández A, et al. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A. 1999;96:248–53. https://doi.org/10.1073/pnas.96.1.248.
Marcheva B, Perelis M, Weidemann BJ, et al. A role for alternative splicing in circadian control of exocytosis and glucose homeostasis. Genes Dev. 2020;34:1089–105. https://doi.org/10.1101/GAD.338178.120.
Liang T, Qin T, Kang F, et al. SNAP23 depletion enables more SNAP25/calcium channel excitosome formation to increase insulin exocytosis in type 2 diabetes. JCI Insight. 2020;5. https://doi.org/10.1172/jci.insight.129694.
Wang X, Fu G, Wen J, et al. Membrane location of syntaxin-binding protein 1 is correlated with poor prognosis of lung adenocarcinoma. Tohoku J Exp Med. 2020;250:263–70. https://doi.org/10.1620/tjem.250.263.
Shim U, Kim HN, Lee H, et al. Pathway analysis based on a genome-wide association study of polycystic ovary syndrome. PLoS One. 2015;10. https://doi.org/10.1371/journal.pone.0136609.
Olesen C, Nyeng P, Kalisz M, et al. Global gene expression analysis in fetal mouse ovaries with and without meiosis and comparison of selected genes with meiosis in the testis. Cell Tissue Res. 2007;328:207–21. https://doi.org/10.1007/s00441-006-0205-5.
Liu K, Zhang H, Risal S, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–8. https://doi.org/10.1016/j.cub.2014.09.023.
Lim JJ, Lima PDA, Salehi R, et al. Regulation of androgen receptor signaling by ubiquitination during folliculogenesis and its possible dysregulation in polycystic ovarian syndrome. Sci Rep. 2017;7. https://doi.org/10.1038/s41598-017-09880-0.
Williams CJ, Erickson GF. Morphology and Physiology of the Ovary. [Updated 2012 Jan 30]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278951/.
Tan J, Wen X-Y, Su Q, et al. Reduced expression of SCF in serum and follicle from patients with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2016;20:5049–57.
Toonen RF, Kochubey O, de Wit H, et al. Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J. 2006;25:3725–37. https://doi.org/10.1038/sj.emboj.7601256.
Funding
This study was supported by Hacettepe University Scientific Research Projects Coordination Unit (Grant numbers: TSA-2019-18196).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SE, SZ, LKS, GB, and SFM. The first draft of the manuscript was written by SE and SFM. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. GB: data collection; SE: project development, protocol, data analysis, manuscript writing; SFM: supervision, project development, protocol, data management and analysis, manuscript editing; LKS: protocol, data collection; SZ: protocol, data analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. Non-interventional Clinical Research Ethics Board of Hacettepe University provided approval for conducting this study (GO 19/678, 2019/16-09).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erol, S., Zırh, S., Bozdag, G. et al. In vitro evaluation of exocytosis-associated SNARE molecules in human granulosa cells in polycystic ovary syndrome. J Assist Reprod Genet 41, 49–61 (2024). https://doi.org/10.1007/s10815-023-02967-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02967-w