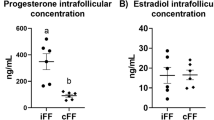
Abstract
The cumulus-oocyte complex (COC) matrix plays a critical role in the ovulation and fertilization process and a major predictor of oocyte quality. Proteomics studies of follicular fluid showed differential expression of COC matrix proteins in women with polycystic ovary syndrome (PCOS), indicating altered COC matrix in these women. In the present study, we aimed to understand COC matrix gene induction in humans and its probable dysfunction in women with PCOS. Animal studies have shown that amphiregulin (AREG) and growth differentiation factor-9 (GDF-9) are important in the induction of COC matrix genes which are involved in cumulus expansion. The effects of AREG and GDF-9 on expression of tumor necrosis factor alpha induced protein 6 (TNFAIP6) and hyaluronan synthase 2 (HAS2) on human cumulus granulosa cells (CGCs) and murine COC expansion were evaluated. Further time-dependent effects of growth factor supplementation on these gene expressions in CGCs from PCOS and control women were compared. Follicular fluid from PCOS showed reduced COC matrix expansion capacity, using murine COCs. Expression of COC matrix genes TNFAIP6 and HAS2 were significantly reduced in CGCs of PCOS. Treatment of CGCs with AREG and GDF-9 together induced expression of both these genes in controls and could only restore HAS2 but not TNFAIP6 expression in PCOS. Our results suggest that the reduced potential of follicular fluid to support COC expansion, altered expression of structural constituents, and intrinsic defects in granulosa cells of women with PCOS may contribute to the aberrant COC organization and expansion in PCOS, thus affecting fertilization.




Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Code Availability
Not applicable.
References
Zhuo L, Kimata K. Cumulus oophorus extracellular matrix: its construction and regulation. Cell Struct Funct. 2001;26(4):189–96. http://www.ncbi.nlm.nih.gov/pubmed/11699635.
Camaioni A, et al. Proteoglycans and proteins in the extracellular matrix of mouse cumulus cell-oocyte complexes. Arch Biochem Biophys. 1996;325(2):190–8. https://doi.org/10.1006/abbi.1996.0024.
Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312. https://doi.org/10.1093/humupd/dml062.
Yokoo M, Sato E. Cumulus-oocyte complex interactions during oocyte maturation. Int Rev Cytol. 2004;235:251–91. https://doi.org/10.1016/S0074-7696(04)35006-0.
Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24(4):217–27. https://doi.org/10.1055/s-2006-948551.
Russell DL, et al. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278(43):42330–9. https://doi.org/10.1074/jbc.M300519200.
Brown HM, et al. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83(4):549–57. https://doi.org/10.1095/biolreprod.110.084434.
Fulop C, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130(10):2253–61. http://www.ncbi.nlm.nih.gov/pubmed/12668637.
Zhuo L, et al. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem. 2001;276(11):7693–6. https://doi.org/10.1074/jbc.C000899200.
Varani S, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16(6):1154–67. https://doi.org/10.1210/mend.16.6.0859.
Shindo T, et al. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 2000;105(10):1345–52. https://doi.org/10.1172/JCI8635.
Yokoo M, Kimura N, Sato E. Induction of oocyte maturation by hyaluronan-CD44 interaction in pigs. J Reprod Dev. 2010;56(1):15–9. https://doi.org/10.1262/jrd.09-173e.
Adriaenssens T, et al. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25(5):1259–70. https://doi.org/10.1093/humrep/deq049.
Wathlet S, et al. Pregnancy prediction in single embryo transfer cycles after ICSI using QPCR: validation in oocytes from the same cohort. PLoS ONE. 2013;8(4):e54226. https://doi.org/10.1371/journal.pone.0054226.
Wathlet S, et al. Cumulus cell gene expression predicts better cleavage-stage embryo or blastocyst development and pregnancy for ICSI patients. Hum Reprod. 2011;26(5):1035–51. https://doi.org/10.1093/humrep/der036.
Shimada M, et al. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352–65. https://doi.org/10.1210/me.2005-0504.
Singh B, Armstrong DT. Insulin-like growth factor-1, a component of serum that enables porcine cumulus cells to expand in response to follicle-stimulating hormone in vitro. Biol Reprod. 1997;56(6):1370–5. http://www.ncbi.nlm.nih.gov/pubmed/9166687.
Liu Z, et al. Interleukin-6: an autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology. 2009;150(7):3360–8. https://doi.org/10.1210/en.2008-1532.
Peralta OA, et al. Granulocyte-macrophage colony stimulating factor (GM-CSF) enhances cumulus cell expansion in bovine oocytes. Reprod Biol Endocrinol. 2013;11:55. https://doi.org/10.1186/1477-7827-11-55.
Panigone S, et al. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22(4):924–36. https://doi.org/10.1210/me.2007-0246.
Park JY, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–4. https://doi.org/10.1126/science.1092463.
Ashkenazi H, et al. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146(1):77–84. https://doi.org/10.1210/en.2004-0588.
Nautiyal J, et al. The nuclear receptor cofactor receptor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte complex expansion in the mouse ovary. Endocrinology. 2010;151(6):2923–32. https://doi.org/10.1210/en.2010-0081.
Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120(4):963–72. https://doi.org/10.1172/JCI41350.
Dragovic RA, et al. Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology. 2005;146(6):2798–806. https://doi.org/10.1210/en.2005-0098.
VarnosfaderaniSh R, et al. Importance of the GDF9 signaling pathway on cumulus cell expansion and oocyte competency in sheep. Theriogenology. 2013;80(5):470–8. https://doi.org/10.1016/j.theriogenology.2013.05.009.
Nagyova E, et al. Activation of cumulus cell SMAD2/3 and epidermal growth factor receptor pathways are involved in porcine oocyte-cumulus cell expansion and steroidogenesis. Mol Reprod Dev. 2011;78(6):391–402. https://doi.org/10.1002/mrd.21312.
Sasseville M, et al. Growth differentiation factor 9 signaling requires ERK1/2 activity in mouse granulosa and cumulus cells. J Cell Sci. 2010;123(Pt 18):3166–76. https://doi.org/10.1242/jcs.063834.
Revelli A, et al. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. https://doi.org/10.1186/1477-7827-7-40.
Ambekar AS, et al. Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. J Proteomics. 2013;87:68–77. https://doi.org/10.1016/j.jprot.2013.05.017.
Hung WT, et al. Extracellular vesicles from bovine follicular fluid support cumulus expansion. Biol Reprod. 2015;93(5):117. https://doi.org/10.1095/biolreprod.115.132977.
Chang RJ, Cook-Andersen H. Disordered follicle development. Mol Cell Endocrinol. 2013;373(1–2):51–60. https://doi.org/10.1016/j.mce.2012.07.011.
Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367–78. https://doi.org/10.1093/humupd/dmn015.
Balen AH, et al. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum Reprod. 1993;8(6):959–64. http://www.ncbi.nlm.nih.gov/pubmed/8345091.
Kodama H, et al. High incidence of embryo transfer cancellations in patients with polycystic ovarian syndrome. Hum Reprod. 1995;10(8):1962–7. http://www.ncbi.nlm.nih.gov/pubmed/8567823.
Ludwig M, et al. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod. 1999;14(2):354–8. http://www.ncbi.nlm.nih.gov/pubmed/10099978.
Plachot M, et al. [Oocyte and embryo quality in polycystic ovary syndrome]. Gynecol Obstet Fertil. 2003;31(4):350–4. https://doi.org/10.1016/s1297-9589(03)00059-6.
Heijnen EM, et al. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(1):13–21. https://doi.org/10.1093/humupd/dmi036.
Kenigsberg S, et al. Gene expression microarray profiles of cumulus cells in lean and overweight-obese polycystic ovary syndrome patients. Mol Hum Reprod. 2009;15(2):89–103. https://doi.org/10.1093/molehr/gan082.
Wood JR, et al. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278(29):26380–90. https://doi.org/10.1074/jbc.M300688200.
Haouzi D, et al. Altered gene expression profile in cumulus cells of mature MII oocytes from patients with polycystic ovary syndrome. Hum Reprod. 2012;27(12):3523–30. https://doi.org/10.1093/humrep/des325.
Ambekar AS, et al. Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab. 2015;100(2):744–53. https://doi.org/10.1210/jc.2014-2086.
Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. https://doi.org/10.1093/humrep/deh098.
Patil K, Hinduja I, Mukherjee S. Alteration in angiogenic potential of granulosa-lutein cells and follicular fluid contributes to luteal defects in polycystic ovary syndrome. Hum Reprod. 2021;36(4):1052–64. https://doi.org/10.1093/humrep/deaa351.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. http://www.ncbi.nlm.nih.gov/pubmed/18546601.
Hsieh M, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27(5):1914–24. https://doi.org/10.1128/MCB.01919-06.
Zamah AM, et al. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010;25(10):2569–78. https://doi.org/10.1093/humrep/deq212.
Conti M, et al. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356(1–2):65–73. https://doi.org/10.1016/j.mce.2011.11.002.
Haouzi D, et al. LH/hCGR gene expression in human cumulus cells is linked to the expression of the extracellular matrix modifying gene TNFAIP6 and to serum estradiol levels on day of hCG administration. Hum Reprod. 2009;24(11):2868–78. https://doi.org/10.1093/humrep/dep263.
Scarchilli L, et al. PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem. 2007;282(41):30161–70. https://doi.org/10.1074/jbc.M703738200.
Salustri A, et al. Hyaluronan and proteoglycans in ovarian follicles. Hum Reprod Update. 1999;5(4):293–301. http://www.ncbi.nlm.nih.gov/pubmed/10465521.
Jansen E, et al. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol. 2004;18(12):3050–63. https://doi.org/10.1210/me.2004-0074.
Du C, et al. FGF2/FGFR signaling promotes cumulus-oocyte complex maturation in vitro. Reproduction. 2021;161(2):205–14. https://doi.org/10.1530/REP-20-0264.
Teixeira Filho FL, et al. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1337–44. https://doi.org/10.1210/jcem.87.3.8316.
Wei LN, et al. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31(11):1483–90. https://doi.org/10.1007/s10815-014-0319-8.
Vitt UA, et al. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod. 2002;67(2):473–80. https://doi.org/10.1095/biolreprod67.2.473.
de Resende LO, et al. Single-cell expression analysis of BMP15 and GDF9 in mature oocytes and BMPR2 in cumulus cells of women with polycystic ovary syndrome undergoing controlled ovarian hyperstimulation. J Assist Reprod Genet. 2012;29(10):1057–65. https://doi.org/10.1007/s10815-012-9825-8.
Prochazka R, Blaha M, Nemcova L. Signaling pathways regulating FSH- and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig. Reproduction. 2012;144(5):535–46. https://doi.org/10.1530/REP-12-0191.
Zhang YE. Non-Smad signaling pathways of the TGF-beta family. Cold Spring Harb Perspect Biol. 2017;9(2):a022129. https://doi.org/10.1101/cshperspect.a022129.
Acknowledgements
The authors would like to thank all study participants, late Dr. Kusum Zaveri, Dr. Veena Bangera, Ms. Leena Mukadam, and Ms. Pushpa Dharwadkar (P.D. Hinduja National Hospital and Medical Research Centre, Mumbai) for collecting samples and providing data records of OPU. The authors appreciate Ms. Aditi Ambekar for her help in designing the study. The authors acknowledge NIRRH (RA/415/09-2016) and ICMR for providing necessary support. We would like to acknowledge the financial assistance provided by CSIR, Government of India, to KP for pursuing her doctoral studies.
Funding
This study was partially funded by the Department of Biotechnology (DBT), Government of India (BT/PR10574/MED/12/394/2008).
Author information
Authors and Affiliations
Contributions
SM: concept and design, interpretation of the data, and final approval for manuscript submission. KP: design and completion of the experiments, analysis of the results, and drafting of the manuscript. GS: carried out animal experiments. IH: provided clinical samples and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study has been approved by Institutional Ethics Committee and written informed consents were obtained from all study participants. This study was also approved by Animal Ethics Committee of the institute. All animal experiments were in accordance with institutional ethics committee guides. Ethics approval number is 139/2007.
Consent for Publication
All authors consent to the publication of this manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patil, K., Shinde, G., Hinduja, I. et al. Compromised Cumulus-Oocyte Complex Matrix Organization and Expansion in Women with PCOS. Reprod. Sci. 29, 836–848 (2022). https://doi.org/10.1007/s43032-021-00775-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00775-0