Abstract
Purpose
We hypothesized that immature oocytes are associated with impaired energy production in surrounding granulosa cells (GCs) in polycystic ovary syndrome (PCOS). Thus, this study investigated mitochondrial function, determined expression of glycolytic regulatory enzymes, and measured ATP levels in GCs of PCOS patients.
Methods
GCs were isolated from forty-five PCOS patients and 45 control women. Intracellular concentration of reactive oxygen species (ROS), mitochondrial membrane potential (Δψm), the rate of glycolysis, total antioxidant capacity (TAC), activities of catalase (CAT) and superoxide dismutase (SOD), and ATP level were measured in GCs. The gene expression and protein levels of glycolytic enzymes (hexokinase, muscular phosphofructokinase, platelet derived phosphofructokinase, and muscular pyruvate kinase) were determined. Association of GC energy level with oocyte maturation was further validated by measuring glycolysis rate and ATP level in GCs isolated from mature and immature follicles from new set of fifteen PCOS patients and 15 controls.
Results
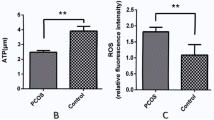
PCOS patients showed higher ROS level, decreased TAC, reduced CAT and SOD activities, and lower Δψm together with reduced expression of key glycolytic enzymes. ATP concentration and biochemical pregnancy were lower in PCOS compared with control group. ATP levels were found to be significantly correlated with ROS and Δψm (r = − 0.624 and r = 0.487, respectively). GCs isolated from immature follicles had significantly lower ATP levels and rate of glycolysis compared with the GCs separated from mature follicles in both PCOS patients and control.
Conclusion
Declined energy due to the mitochondrial dysfunction and restrained glycolysis in GCs is associated with the immature oocytes and lower biochemical pregnancy in PCOS.
Graphical Abstract








Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Forslund M, Landin-Wilhelmsen K, Trimpou P, Schmidt J, Brännström M, Dahlgren E. Type 2 diabetes mellitus in women with polycystic ovary syndrome during a 24-year period: importance of obesity and abdominal fat distribution. Human Reproduction Open. 2020;2020(1):hoz042.
Fitzgerald S, DiVasta A, Gooding H. An update on PCOS in adolescents. Curr Opin Pediatr. 2018;30(4):459–65.
Chronowska E. High-throughput analysis of ovarian granulosa cell transcriptome. BioMed Res Int. 2014;2014:213570.
Vartanyan E, Tsaturova K, Devyatova E, Mikhaylyukova A, Levin V, Petuhova N, et al. Improvement in quality of oocytes in polycystic ovarian syndrome in programs of in vitro fertilization. Gynecol Endocrinol. 2017;33(sup1):8–11.
Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139(4):685.
Wang J, Wu X. The effects of mitochondrial dysfunction on energy metabolism switch by HIF-1α signalling in granulosa cells of polycystic ovary syndrome. Endokrynol Pol. 2020;71(2):134–45.
Zhang S, Tu H, Yao J, Le J, Jiang Z, Tang Q, et al. Combined use of diane-35 and metformin improves the ovulation in the PCOS rat model possibly via regulating glycolysis pathway. Reprod Biol Endocrinol. 2020;18(1):1–11.
Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16(1):1–18.
Hou G-X, Liu P-P, Zhang S, Yang M, Liao J, Yang J, et al. Elimination of stem-like cancer cell side-population by auranofin through modulation of ROS and glycolysis. Cell Death Dis. 2018;9(2):1–15.
Munakata Y, Ichinose T, Ogawa K, Itami N, Tasaki H, Shirasuna K, et al. Relationship between the number of cells surrounding oocytes and energy states of oocytes. Theriogenology. 2016;86(7):1789-98.e1.
ESHRE TR, Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81(1):19–25
Nguyen TT, Doan HT, Quan LH, Lam NM. Effect of letrozole for ovulation induction combined with intrauterine insemination on women with polycystic ovary syndrome. Gynecol Endocrinol. 2020;36(10):860–3.
Jafarzadeh H, Nazarian H, Ghaffari Novin M, Shams Mofarahe Z, Eini F, Piryaei A. Improvement of oocyte in vitro maturation from mice with polycystic ovary syndrome by human mesenchymal stromal cell–conditioned media. J Cell Biochem. 2018;119(12):10365–75.
Kansaku K, Itami N, Kawahara-Miki R, Shirasuna K, Kuwayama T, Iwata H. Differential effects of mitochondrial inhibitors on porcine granulosa cells and oocytes. Theriogenology. 2017;103:98–103.
Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):32–42.
Xie H-L, Wang Y-B, Jiao G-Z, Kong D-L, Li Q, Li H, et al. Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes. Sci Rep. 2016;6(1):1–11.
Li D, Wang X, Li G, Dang Y, Zhao S, Qin Y. LncRNA ZNF674-AS1 regulates granulosa cell glycolysis and proliferation by interacting with ALDOA. Cell death discovery. 2021;7(1):1–12.
Sugiyama M, Sumiya M, Shirasuna K, Kuwayama T, Iwata H. Addition of granulosa cell mass to the culture medium of oocytes derived from early antral follicles increases oocyte growth, ATP content, and acetylation of H4K12. Zygote. 2016;24(6):848–56.
Lai Q, Xiang W, Li Q, Zhang H, Li Y, Zhu G, et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front Med. 2018;12(5):518–24.
Huang M, Huang M, Li X, Liu S, Fu L, Jiang X, et al. Bisphenol A induces apoptosis through GPER-dependent activation of the ROS/Ca2+-ASK1-JNK pathway in human granulosa cell line KGN. Ecotoxicol Environ Saf. 2021;208: 111429.
Lin X, Dai Y, Tong X, Xu W, Huang Q, Jin X, et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. 2020;30: 101431.
Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab. 2013;17(6):838–50.
Simsek-Duran F, Li F, Ford W, Swanson RJ, Jones HW Jr, Castora FJ. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE. 2013;8(5): e64955.
Rambags B, Van Boxtel D, Tharasanit T, Lenstra J, Colenbrander B, Stout T. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology. 2014;81(7):959–65.
Lemasters JJ, Qian T, Bradham CA, Brenner DA, Cascio WE, Trost LC, et al. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr. 1999;31(4):305–19.
Dumesic DA, Guedikian AA, Madrigal VK, Phan JD, Hill DL, Alvarez JP, et al. Cumulus cell mitochondrial resistance to stress in vitro predicts oocyte development during assisted reproduction. J Clin Endocrinol Metab. 2016;101(5):2235–45.
Liu Y, Han M, Li X, Wang H, Ma M, Zhang S, et al. Age-related changes in the mitochondria of human mural granulosa cells. Hum Reprod. 2017;32(12):2465–73.
Zhang J, Bao Y, Zhou X, Zheng L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol. 2019;17(1):1–15.
Cao J, Huo P, Cui K, Wei H, Cao J, Wang J, et al. Follicular fluid-derived exosomal miR-143-3p/miR-155-5p regulate follicular dysplasia by modulating glycolysis in granulosa cells in polycystic ovary syndrome. Cell Commun Signal. 2022;20(1):1–17.
Fontana J, Martinkova S, Jaroslav P, Žalmanová T, Trnka J. Metabolic cooperation in the ovarian follicle. Physiol Res. 2020;69(1):33.
Hoque SM, Kawai T, Zhu Z, Shimada M. Mitochondrial protein turnover is critical for granulosa cell proliferation and differentiation in antral follicles. J Endocrine Soc. 2019;3(2):324–39.
Benkhalifa M, Ferreira YJ, Chahine H, Louanjli N, Miron P, Merviel P, et al. Mitochondria: participation to infertility as source of energy and cause of senescence. Int J Biochem Cell Biol. 2014;55:60–4.
Munakata Y, Kawahara-Miki R, Shiratsuki S, Tasaki H, Itami N, Shirasuna K, Kuwayama T, Iwata H. Gene expression patterns in granulosa cells and oocytes at various stages of follicle development as well as in in vitro grown oocyte-and-granulosa cell complexes. J Reprod Dev. 2016;62(4):359–66.
Liang A, Le Huang, Liu H, He W, Lei X, Li M, et al. Resveratrol improves follicular development of PCOS rats by regulating the glycolytic pathway. Molec Nutr Food Res. 2021;65(24):2100457.
Zhang X, Zhang W, Wang Z, Zheng N, Yuan F, Li B, et al. Enhanced glycolysis in granulosa cells promotes the activation of primordial follicles through mTOR signaling. Cell Death Dis. 2022;13(1):1–14.
Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, et al. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005;20(2):373–81.
Rodríguez-Nuevo A, Torres-Sanchez A, Duran JM, De Guirior C, Martínez-Zamora MA, Böke E. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature. 2022;607(7920):756–61.
Pei C-Z, Jin L, Baek K-H. Pathogenetic analysis of polycystic ovary syndrome from the perspective of omics. Biomed Pharmacother. 2021;142: 112031.
Zhao R, Jiang Y, Zhao S, Zhao H. Multiomics analysis reveals molecular abnormalities in granulosa cells of women with polycystic ovary syndrome. Front Genet. 2021;12: 648701.
Gupta S, Ghulmiyyah J, Sharma R, Halabi J, Agarwal A. Power of proteomics in linking oxidative stress and female infertility. BioMed Res Int. 2014;2014:916212.
Min Z, Long X, Zhao H, Zhen X, Li R, Li M, et al. Protein lysine acetylation in ovarian granulosa cells affects metabolic homeostasis and clinical presentations of women with polycystic ovary syndrome. Front Cell Dev Biol. 2020;8: 567028.
Kaur S, Archer KJ, Devi MG, Kriplani A, Strauss JF III, Singh R. Differential gene expression in granulosa cells from polycystic ovary syndrome patients with and without insulin resistance: identification of susceptibility gene sets through network analysis. J Clin Endocrinol Metab. 2012;97(10):E2016–21.
Zhang Y, Liu L, Yin T-L, Yang J, Xiong C-L. Follicular metabolic changes and effects on oocyte quality in polycystic ovary syndrome patients. Oncotarget. 2017;8(46):80472.
Acknowledgements
The authors wish to acknowledge Hamadan University of Medical Sciences for the financial support of the study as PhD thesis project (Project NO: 9809056544).
Author information
Authors and Affiliations
Contributions
IK and SM contributed to the conceptualization and designing of the study. MSF and IA contributed in provision of materials, collaborated in patient enrolment, and managed sample collection. SM carried out all laboratory works and IK, HT, and JK supervised the experiments. SM and EA collected the data. IK, SM, and EA performed statistical analysis. IK and SM wrote the first draft of the manuscript, and all authors reviewed the manuscript. IK edited the manuscript, and the revised version was approved by all authors. IK supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mazloomi, S., Farimani, M.S., Tavilani, H. et al. Granulosa cells from immature follicles exhibit restricted glycolysis and reduced energy production: a dominant problem in polycystic ovary syndrome. J Assist Reprod Genet 40, 343–359 (2023). https://doi.org/10.1007/s10815-022-02676-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02676-w