Abstract
Purpose
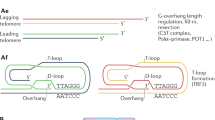
Unlike other cells in the body, in sperm, telomere length (TL) increases with age. TL can regulate nearby genes, and the subtelomeric region is rich in retrotransposons. We hypothesized that age-related telomere lengthening in sperm might suppress Long Interspersed Element 1 (LINE-1/L1), the only competent retrotransposon in humans.
Methods
We measured L1 copy number (L1-CN) and sperm telomere length (STL) from young and older men to evaluate the relationship between age, TL and L1-CN. We also evaluated L1-CN and TL in individual sperm to determine whether these variables influence sperm morphology. STL was assayed by Multiplex quantitative polymerase chain reaction method (mmqPCR) and L1-CN by Quantitative polymerase chain reaction (qPCR).
Results
We found that STL increased, and L1-CN decreased significantly with paternal age. STL in normal single sperm was significantly higher than in abnormal sperm. L1-CN did not differ between normal and abnormal sperm. Furthermore, morphologically normal sperm have longer telomeres than abnormal sperm.
Conclusions
Elongation of telomeres in the male germline could repress retrotransposition, which tends to increase with cellular aging. More studies in larger cohorts across a wide age span are needed to confirm our conclusions and explore their biological and clinical significance.




Similar content being viewed by others
Data availability
The data underlying this study will be shared on reasonable request to the corresponding author.
Abbreviations
- CoV:
-
Coefficient of variation
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylenediaminetetraacetic acid
- gDNA:
-
Genomic DNA
- ICSI:
-
Intracytoplasmic sperm injection
- IRB:
-
Institutional Review Board
- IQR:
-
Interquartile range
- L1:
-
Long interspersed element 1
- L1-CN:
-
L1 copy number
- Long Interspersed Element 1:
-
LINE-1/L1
- mmqPCR:
-
Multiplex quantitative polymerase chain reaction method
- non-LTR:
-
TE-non-long terminal repeat
- NTC:
-
Non-template control
- NYU:
-
New York University
- NYUFC:
-
New York University Langone Fertility Center
- ORFs:
-
Open reading frames
- piRNA:
-
PIWI-interacting RNA
- PTC:
-
Positive control
- PVP:
-
Polyvinylpyrrolidone
- qPCR:
-
Quantitative polymerase chain reaction
- rDNA:
-
Recombinant DNA
- RT:
-
Reverse transcriptase
- RUES:
-
Rockefeller University
- SC-qPCR:
-
Single-cell telomere length assay
- STL:
-
Sperm telomere length
- TEs:
-
Transposable elements
- TL:
-
Telomere length
- TPE:
-
Telomere position effect
- WHO:
-
World Health Organization
References
Mita P, Boeke JD. How retrotransposons shape genome regulation. Curr Opin Genet Dev. 2016;37:90–100. https://doi.org/10.1016/j.gde.2016.01.001.
Belan E. LINEs of evidence: noncanonical DNA replication as an epigenetic determinant. Biol Direct. 2013;8:22. https://doi.org/10.1186/1745-6150-8-22.
Terry DM, Devine SE. Aberrantly High Levels of Somatic LINE-1 Expression and Retrotransposition in Human Neurological Disorders. Front Genet. 2019;10:1244. https://doi.org/10.3389/fgene.2019.01244.
Kuroki R, Murata Y, Fuke S, Nakachi Y, Nakashima J, Kujoth GC, et al. Establishment of Quantitative PCR Assays for Active Long Interspersed Nuclear Element-1 Subfamilies in Mice and Applications to the Analysis of Aging-Associated Retrotransposition. Front Genet. 2020;11:519206. https://doi.org/10.3389/fgene.2020.519206.
Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–93. https://doi.org/10.1126/science.1063443.
Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc Lond B Biol Sci. 2013;368(1609):20110330. https://doi.org/10.1098/rstb.2011.0330.
Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol. 2002;241(1):172–82. https://doi.org/10.1006/dbio.2001.0501.
Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, et al. L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev. 2009;23(11):1303–12. https://doi.org/10.1101/gad.1803909.
Solyom S, Kazazian HH Jr. Mobile elements in the human genome: implications for disease. Genome Med. 2012;4(2):12. https://doi.org/10.1186/gm311.
Kazazian HH Jr, Moran JV. Mobile DNA in Health and Disease. N Engl J Med. 2017;377(4):361–70. https://doi.org/10.1056/NEJMra1510092.
Burns KH, Boeke JD. Human transposon tectonics. Cell. 2012;149(4):740–52. https://doi.org/10.1016/j.cell.2012.04.019.
Kazazian HH Jr. Genetics L1 retrotransposons shape the mammalian genome. Science. 2000;289(5482):1152–3. https://doi.org/10.1126/science.289.5482.1152.
Kohlrausch FB, Berteli TS, Wang F, Navarro PA, Keefe DL. Control of LINE-1 Expression Maintains Genome Integrity in Germline and Early Embryo Development. Reprod Sci. 2022;29(2):328–40. https://doi.org/10.1007/s43032-021-00461-1.
Kazazian HH. Retrotransposon insertions in germ cells and somatic cells. Dev Biol (Basel). 2001;106:307–13.
Kazazian HH Jr. Mobile elements: drivers of genome evolution. Science. 2004;303(5664):1626–32. https://doi.org/10.1126/science.1089670.
Gorbunova V, Seluanov A, Mita P, McKerrow W, Fenyö D, Boeke JD, et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature. 2021;596(7870):43–53. https://doi.org/10.1038/s41586-021-03542-y.
Spadafora C. Sperm-Mediated Transgenerational Inheritance. Front Microbiol. 2017;8:2401. https://doi.org/10.3389/fmicb.2017.02401.
Smith K, Spadafora C. Sperm-mediated gene transfer: applications and implications. BioEssays. 2005;27(5):551–62. https://doi.org/10.1002/bies.20211.
Vitullo P, Sciamanna I, Baiocchi M, Sinibaldi-Vallebona P, Spadafora C. LINE-1 retrotransposon copies are amplified during murine early embryo development. Mol Reprod Dev. 2012;79(2):118–27. https://doi.org/10.1002/mrd.22003.
Newkirk SJ, Lee S, Grandi FC, Gaysinskaya V, Rosser JM, Vanden Berg N, et al. Intact piRNA pathway prevents L1 mobilization in male meiosis. Proc Natl Acad Sci USA. 2017;114(28):E5635–44. https://doi.org/10.1073/pnas.1701069114.
Bray I, Gunnell D, Davey SG. Advanced paternal age: how old is too old? J Epidemiol Community Health. 2006;60(10):851–3. https://doi.org/10.1136/jech.2005.045179.
Biron-Shental T, Wiser A, Hershko-Klement A, Markovitch O, Amiel A, Berkovitch A. Sub-fertile sperm cells exemplify telomere dysfunction. J Assist Reprod Genet. 2018;35(1):143–8. https://doi.org/10.1007/s10815-017-1029-9.
Vasilopoulos E, Fragkiadaki P, Kalliora C, Fragou D, Docea AO, Vakonaki E, et al. The association of female and male infertility with telomere length (Review). Int J Mol Med. 2019;44(2):375–89. https://doi.org/10.3892/ijmm.2019.4225.
Yang Q, Zhang N, Zhao F, Zhao W, Dai S, Liu J, et al. Processing of semen by density gradient centrifugation selects spermatozoa with longer telomeres for assisted reproduction techniques. Reprod Biomed Online. 2015;31(1):44–50. https://doi.org/10.1016/j.rbmo.2015.02.016.
Anifandis G, Samara M, Simopoulou M, Messini CI, Chatzimeletiou K, Thodou E, et al. Insights into the Role of Telomeres in Human Embryological Parameters. Opinions Regarding IVF. J Dev Biol. 2021;9(4). https://doi.org/10.3390/jdb9040049.
Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian J Androl. 2012;14(1):6–13. https://doi.org/10.1038/aja.2011.58.
Pizzol D, Ferlin A, Garolla A, Lenzi A, Bertoldo A, Foresta C. Genetic and molecular diagnostics of male infertility in the clinical practice. Front Biosci (Landmark Ed). 2014;19(2):291–303. https://doi.org/10.2741/4208.
Tahamtan S, Tavalaee M, Izadi T, Barikrow N, Zakeri Z, Lockshin RA, et al. Reduced sperm telomere length in individuals with varicocele is associated with reduced genomic integrity. Sci Rep. 2019;9(1):4336. https://doi.org/10.1038/s41598-019-40707-2.
Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013;287(4):803–7. https://doi.org/10.1007/s00404-012-2632-8.
Lafuente R, Bosch-Rue E, Ribas-Maynou J, Alvarez J, Brassesco C, Amengual MJ, et al. Sperm telomere length in motile sperm selection techniques: A qFISH approach. Andrologia. 2018;50(2). https://doi.org/10.1111/and.12840.
Amirzadegan M, Sadeghi N, Tavalaee M, Nasr-Esfahani MH. Analysis of leukocyte and sperm telomere length in oligozoospermic men. Andrologia. 2021;53(10):e14204. https://doi.org/10.1111/and.14204.
Thilagavathi J, Venkatesh S, Dada R. Telomere length in reproduction. Andrologia. 2013;45(5):289–304. https://doi.org/10.1111/and.12008.
Rocca MS, Speltra E, Menegazzo M, Garolla A, Foresta C, Ferlin A. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum Reprod. 2016;31(6):1158–63. https://doi.org/10.1093/humrep/dew061.
Siderakis M, Tarsounas M. Telomere regulation and function during meiosis. Chromosome Res. 2007;15(5):667–79. https://doi.org/10.1007/s10577-007-1149-7.
Darmishonnejad Z, Tavalaee M, Izadi T, Tanhaei S, Nasr-Esfahani MH. Evaluation of sperm telomere length in infertile men with failed/low fertilization after intracytoplasmic sperm injection. Reprod Biomed Online. 2019;38(4):579–87. https://doi.org/10.1016/j.rbmo.2018.12.022.
Powell D, Cran DG, Jennings C, Jones R. Spatial organization of repetitive DNA sequences in the bovine sperm nucleus. J Cell Sci. 1990;97(Pt 1):185–91. https://doi.org/10.1242/jcs.97.1.185.
Antunes DMF, Kalmbach KH, Wang F, Dracxler RC, Seth-Smith ML, Kramer Y, et al. A single-cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet. 2015;32(11):1685–90. https://doi.org/10.1007/s10815-015-0574-3.
Baur JA, Zou Y, Shay JW, Wright WE. Telomere position effect in human cells. Science. 2001;292(5524):2075–7. https://doi.org/10.1126/science.1062329.
Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;2(3):245–61. https://doi.org/10.1016/s1568-1637(03)00010-2.
WHO. World Health Organization laboratory manual for the examination and processing of human semen. 5th ed: World Health Organization; 2010.
Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. https://doi.org/10.1093/nar/gkn1027.
Wang F, Pan X, Kalmbach K, Seth-Smith ML, Ye X, Antumes DM, et al. Robust measurement of telomere length in single cells. Proc Natl Acad Sci U S A. 2013;110(21):E1906–12. https://doi.org/10.1073/pnas.1306639110.
Zhang P, Ludwig AK, Hastert FD, et al. L1 retrotransposition is activated by Ten-eleven-translocation protein 1 and repressed by methyl-CpG binding proteins. Nucleus. 2017;8(5):548–62. https://doi.org/10.1080/19491034.2017.1330238.
Schmidt H, Taubert H, Lange H, Kriese K, Schmitt WD, Hoffmann S, Bartel F, Hauptmann S. Small polydispersed circular DNA contains strains of mobile genetic elements and occurs more frequently in permanent cell lines of malignant tumors than in normal lymphocytes. Oncol Rep. 2009;22(2):393–400.
Macia A, Widmann TJ, Heras SR, Ayllon V, Sanchez L, Benkaddour-Boumzaouad M, Muñoz-Lopez M, Rubio A, Amador-Cubero S, Blanco-Jimenez E, Garcia-Castro J, Menendez P, Ng P, Muotri AR, Goodier JL, Garcia-Perez JL. Engineered LINE-1 retrotransposition in nondividing human neurons. Genome Res. 2017;27(3):335–48. https://doi.org/10.1101/gr.206805.116.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. https://doi.org/10.1038/nprot.2008.73.
Eisenberg DT, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci USA. 2012;109(26):10251–6. https://doi.org/10.1073/pnas.1202092109.
Yang Q, Zhao F, Dai S, Zhang N, Zhao W, Bai R, et al. Sperm telomere length is positively associated with the quality of early embryonic development. Hum Reprod. 2015;30(8):1876–81. https://doi.org/10.1093/humrep/dev144.
Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, et al. Offspring's leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37. https://doi.org/10.1371/journal.pgen.0040037.
Zakian VA. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. https://doi.org/10.1146/annurev.ge.23.120189.003051.
Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21(1):10–4. https://doi.org/10.1071/rd08229.
Riethman H. Human telomere structure and biology. Annu Rev Genomics Hum Genet. 2008;9:1–19. https://doi.org/10.1146/annurev.genom.8.021506.172017.
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–5. https://doi.org/10.1126/science.7605428.
Reig-Viader R, Brieño-Enríquez MA, Khoriauli L, Toran N, Cabero L, Giulotto E, et al. Telomeric repeat-containing RNA and telomerase in human fetal oocytes. Hum Reprod. 2013;28(2):414–22. https://doi.org/10.1093/humrep/des363.
Reig-Viader R, Vila-Cejudo M, Vitelli V, Buscà R, Sabaté M, Giulotto E, et al. Telomeric repeat-containing RNA (TERRA) and telomerase are components of telomeres during mammalian gametogenesis. Biol Reprod. 2014;90(5):103. https://doi.org/10.1095/biolreprod.113.116954.
Richardson SR, Gerdes P, Gerhardt DJ, Sanchez-Luque FJ, Bodea GO, Muñoz-Lopez M, et al. Heritable L1 retrotransposition in the mouse primordial germline and early embryo. Genome Res. 2017;27(8):1395–405. https://doi.org/10.1101/gr.219022.116.
Kordyukova M, Olovnikov I, Kalmykova A. Transposon control mechanisms in telomere biology. Curr Opin Genet Dev. 2018;49:56–62. https://doi.org/10.1016/j.gde.2018.03.002.
Starnes JH, Thornbury DW, Novikova OS, Rehmeyer CJ, Farman ML. Telomere-targeted retrotransposons in the rice blast fungus Magnaporthe oryzae: agents of telomere instability. Genetics. 2012;191(2):389–406. https://doi.org/10.1534/genetics.111.137950.
Higashiyama T, Noutoshi Y, Fujie M, Yamada T. Zepp, a LINE-like retrotransposon accumulated in the Chlorella telomeric region. EMBO J. 1997;16(12):3715–23. https://doi.org/10.1093/emboj/16.12.3715.
Osanai-Futahashi M, Fujiwara H. Coevolution of telomeric repeats and telomeric repeat-specific non-LTR retrotransposons in insects. Mol Biol Evol. 2011;28(11):2983–6. https://doi.org/10.1093/molbev/msr135.
Gladyshev EA, Arkhipova IR. Telomere-associated endonuclease-deficient Penelope-like retroelements in diverse eukaryotes. Proc Natl Acad Sci U S A. 2007;104(22):9352–7. https://doi.org/10.1073/pnas.0702741104.
Morrish TA, Garcia-Perez JL, Stamato TD, Taccioli GE, Sekiguchi J, Moran JV. Endonuclease-independent LINE-1 retrotransposition at mammalian telomeres. Nature. 2007;446(7132):208–12. https://doi.org/10.1038/nature05560.
Beck CR, Garcia-Perez JL, Badge RM, Moran JV. LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet. 2011;12:187–215. https://doi.org/10.1146/annurev-genom-082509-141802.
Saint-Leandre B, Nguyen SC, Levine MT. Diversification and collapse of a telomere elongation mechanism. Genome Res. 2019;29(6):920–31. https://doi.org/10.1101/gr.245001.118.
Lavie L, Kitova M, Maldener E, Meese E, Mayer J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2). J Virol. 2005;79(2):876–83. https://doi.org/10.1128/jvi.79.2.876-883.2005.
Schulz WA, Steinhoff C, Florl AR. Methylation of endogenous human retroelements in health and disease. Curr Top Microbiol Immunol. 2006;310:211–50. https://doi.org/10.1007/3-540-31181-5_11.
Smallwood SA, Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet. 2012;28(1):33–42. https://doi.org/10.1016/j.tig.2011.09.004.
Lazaros L, Kitsou C, Kostoulas C, Bellou S, Hatzi E, Ladias P, et al. Retrotransposon expression and incorporation of cloned human and mouse retroelements in human spermatozoa. Fertil Steril. 2017;107(3):821–30. https://doi.org/10.1016/j.fertnstert.2016.12.027.
Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, et al. L1 retrotransposition in human neural progenitor cells. Nature. 2009;460(7259):1127–31. https://doi.org/10.1038/nature08248.
De Cecco M, Criscione SW, Peckham EJ, Hillenmeyer S, Hamm EA, Manivannan J, et al. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell. 2013;12(2):247–56. https://doi.org/10.1111/acel.12047.
De Cecco M, Ito T, Petrashen AP, Elias AE, Skvir NJ, Criscione SW, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019;566(7742):73–8. https://doi.org/10.1038/s41586-018-0784-9.
Aschacher T, Wolf B, Enzmann F, Kienzl P, Messner B, Sampl S, et al. LINE-1 induces hTERT and ensures telomere maintenance in tumour cell lines. Oncogene. 2016;35(1):94–104. https://doi.org/10.1038/onc.2015.65.
Aschacher T, Wolf B, Aschacher O, Enzmann F, Laszlo V, Messner B, et al. Long interspersed element-1 ribonucleoprotein particles protect telomeric ends in alternative lengthening of telomeres dependent cells. Neoplasia. 2020;22(2):61–75. https://doi.org/10.1016/j.neo.2019.11.002.
Gainetdinov I, Skvortsova Y, Kondratieva S, Funikov S, Azhikina T. Two modes of targeting transposable elements by piRNA pathway in human testis. RNA. 2017;23(11):1614–25. https://doi.org/10.1261/rna.060939.117.
Reznik B, Cincotta SA, Jaszczak RG, Mateo LJ, Shen J, Cao M, et al. Heterogeneity of transposon expression and activation of the repressive network in human fetal germ cells. Development. 2019;146(12). https://doi.org/10.1242/dev.171157.
Acknowledgements
Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code: 001, Brazil; process n. 88887.371487/2019-00 and process n. 88887.597054/2021-00 to TSB), Brazilian National Council for Scientific and Technological Development (CNPq, Brazil; Grant number 204747/2018-0 to FBK) and the Stanley H. Kaplan Fund of the NYU Grossman School of Medicine (to DLK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The New York University Langone Medical Center Institutional Review Board (IRB) approved this study under the IRB number S#16–00154.
Consent to participate
Each participant provided written informed consent.
Consent for publication
The author agree to its submission to the Journal of Assisted Reproduction and Genetics and, if accepted, to its publication in this journal. We warrant that this article is original, does not infringe on any copyright or other proprietary right of any third party, is not under consideration by another journal and has not been previously published. Ethical approval has been sought and obtained as necessary and any conflicts of interest stated.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berteli, T.S., Wang, F., Navarro, P.A. et al. A pilot study of LINE-1 copy number and telomere length with aging in human sperm. J Assist Reprod Genet 40, 1845–1854 (2023). https://doi.org/10.1007/s10815-023-02857-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02857-1