Abstract
Purpose
Polycystic ovary syndrome is a complex heterogeneous endocrine disorder associated with established metabolic abnormalities and is a common cause of infertility in females. Glutathione metabolism in the cumulus cells (CCs) of women with PCOS may be correlated to the quality of oocytes for infertility treatment; therefore, we used a metabolomics approach to examine changes in CCs from women with PCOS and oocyte quality.
Methods
Among 135 women undergoing fertility treatment in the present study, there were 43 women with PCOS and 92 without. CCs were collected from the two groups and levels of pyroglutamic acid were measured using LC–MS/MS followed by qPCR and Western blot analysis to examine genes and proteins involved in pyroglutamic acid metabolism related to glutathione synthesis.
Results
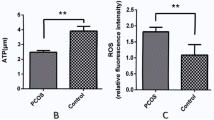
Women with PCOS showed increased levels of l-pyroglutamic acid, l-glutamate, and l-phenylalanine and decreased levels of Cys–Gly and N-acetyl-l-methionine. Gene expression of OPLAH, involved in pyroglutamic synthesis, was significantly increased in women with PCOS compared with those without. Gene expression of GSS was significantly decreased in women with PCOS and synthesis of glutathione synthetase protein was decreased. Expression of nuclear factor erythroid 2-related factor 2, involved in resistance to oxidative stress, was significantly increased in women with PCOS.
Conclusions
CCs of women with PCOS showed high concentrations of pyroglutamic acid and reduced glutathione synthesis, which causes oxidative stress in CCs, suggesting that decreased glutathione synthesis due to high levels of pyroglutamic acid in CCs may be related to the quality of oocytes in women with PCOS.





Similar content being viewed by others
Data availability
Data and material are available offline.
References
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–31.
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97.
Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10.
Mukherjee S, Maitra A. Molecular & genetic factors contributing to insulin resistance in polycystic ovary syndrome. Indian J Med Res. 2010;131(6):743–61.
Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, Sternfeld B, Wellons M, Schwartz SM, Lewis CE. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6.
Dong F, Deng D, Chen H, Cheng W, Li Q, Luo R, Ding S. Serum metabolomics study of polycystic ovary syndrome based on UPLC-QTOF-MS coupled with a pattern recognition approach. Anal Bioanal Chem. 2015;407(16):4683–95.
Zhao X, Xu F, Qi B, Hao S, Li Y, Li Y, Zou L, Lu C, Xu G, Hou L. Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography–mass spectrometry. J Proteome Res. 2014;13(2):1101–11. https://doi.org/10.1021/pr401130w.
Atiomo W, Daykin C. Metabolomic biomarkers in women with polycystic ovary syndrome: a pilot study. Mol Hum Reprod. 2012;18(11):546–53.
Zhao Y, Fu L, Li R, Wang L-N, Yang Y, Liu N-N, Zhang C-M, Wang Y, Liu P, Tu B-B, Zhang X, Qiao J. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10(1):153.
Brinca AT, Ramalhinho AC, Sousa Â, Oliani AH, Breitenfeld L, Passarinha LA, Gallardo E. Follicular Fluid: A powerful tool for the understanding and diagnosis of polycystic ovary syndrome. Biomedicines. 2022;10(6):1254.
Chang AY, Lalia AZ, Jenkins GD, Dutta T, Carter RE, Singh RJ, Nair KS. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metab. 2017;71:52–63.
Zhao H, Zhao Y, Li T, Li M, Li J, Li R, Liu P, Yu Y, Qiao J. Metabolism alteration in follicular niche: the nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic Biol Med. 2015;86:295–307.
Gamarra Y, Santiago FC, Molina-López J, Castaño J, Herrera-Quintana L, Domínguez Á, Planells E. Pyroglutamic acidosis by glutathione regeneration blockage in critical patients with septic shock. Crit Care. 2019;23(1):162.
Palmer BF, Alpern RJ. CHAPTER 12 - Metabolic Acidosis. In: Floege J, Johnson RJ, Feehally J, editors. Comprehensive clinical nephrology. 4th ed. Philadelphia: Mosby; 2010. p. 155–66.
El-Hafidi M, Franco M, Ramírez AR, Sosa JS, Flores JAP, Acosta OL, Salgado MC, Cardoso-Saldaña G. Glycine increases insulin sensitivity and glutathione biosynthesis and protects against oxidative stress in a model of sucrose-induced insulin resistance. Oxid Med Cell Longev. 2018;2018:2101562. https://doi.org/10.1155/2018/2101562.
Wardell RJ, Burrows LA, Myall K, Marsh A. An unusual cause of high anion gap metabolic acidosis: pyroglutamic acidosis. Crit Care. 2012;16(1):P148.
Demiray SB, Goker ENT, Tavmergen E, Yilmaz O, Calimlioglu N, Soykam HO, Oktem G, Sezerman U. Differential gene expression analysis of human cumulus cells. Clin Exp Reprod Med. 2019;46(2):76–86.
ESHRE TR, Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Grøndahl ML, Andersen CY, Bogstad J, Borgbo T, Hartvig Boujida V, Borup R. Specific genes are selectively expressed between cumulus and granulosa cells from individual human pre-ovulatory follicles. Mol Hum Reprod. 2012;18(12):572–84.
Sheikh KD, Khanna S, Byers SW, Fornace AJ Jr, Cheema AK. Small molecule metabolite extraction strategy for improving LC/MS detection of cancer cell metabolome. J Biomol Tech. 2011;22(1):1.
Gao E-M, Turathum B, Wang L, Zhang D, Liu Y-B, Tang R-X, Chian R-C. The differential metabolomes in cumulus and mural granulosa cells from human preovulatory follicles. Reprod Sci. 2022;29(4):1343–56.
Zhang Z, Hong Y, Chen M, Tan N, Liu S, Nie X, Zhou W. Serum metabolomics reveals metabolic profiling for women with hyperandrogenism and insulin resistance in polycystic ovary syndrome. Metabolomics. 2020;16(2):20.
Hou E, Zhao Y, Hang J, Qiao J. Metabolomics and correlation network analysis of follicular fluid reveals associations between l-tryptophan, l-tyrosine and polycystic ovary syndrome. Biomed Chromatogr. 2021;35(3):e4993.
Kumar A, Bachhawat AK. Pyroglutamic acid: throwing light on a lightly studied metabolite. Curr Sci. 2012;288–97.
Jackson AA, Gibson NR, Lu Y, Jahoor F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am J Clin Nutr. 2004;80(1):101–7.
Geenen S, Yates JW, Kenna JG, Bois FY, Wilson ID, Westerhoff HV. Multiscale modelling approach combining a kinetic model of glutathione metabolism with PBPK models of paracetamol and the potential glutathione-depletion biomarkers ophthalmic acid and 5-oxoproline in humans and rats. Integr Biol. 2013;5(6):877–88.
Yu Y-M, Ryan CM, Fei Z-W, Lu X-M, Castillo L, Schultz JT, Tompkins RG, Young VR. Plasma L-5-oxoproline kinetics and whole blood glutathione synthesis rates in severely burned adult humans. Am J Physiol Endocrinol Metab. 2002;282(2):E247–58.
Lord RS. Long-term patterns of urinary pyroglutamic acid in healthy humans. Physiol Rep. 2016;4(4):e12706.
Jackson AA, Badaloo AV, Forrester T, Hibbert J, Persaud C. Urinary excretion of 5-oxoproline (pyroglutamic aciduria) as an index of glycine insufficiency in normal man. Br J Nutr. 1987;58(2):207–14.
Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation1–4. Am J Clin Nutr. 2011;94(3):847–53.
Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–48.
Fatima Q, Amin S, Kawa IA, Jeelani H, Manzoor S, Rizvi SM, Rashid F. Evaluation of antioxidant defense markers in relation to hormonal and insulin parameters in women with polycystic ovary syndrome (PCOS): a case-control study. Diabetes Metab Syndr. 2019;13(3):1957–61.
Liu Y, Yu Z, Zhao S, Cheng L, Man Y, Gao X, Zhao H. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J Assist Reprod Genet. 2021;38(2):471–7.
Assou S, Haouzi D, De Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol Hum Reprod. 2010;16(8):531–8.
Wang L, Guo M, Wang K, Zhang L. Prognostic roles of central carbon metabolism-associated genes in patients with low-grade glioma. Front Genet. 2019;10:831.
Carretero J, Obrador E, Anasagasti MJ, Martin JJ, Vidal-Vanaclocha F, Estrela JM. Growth-associated changes in glutathione content correlate with liver metastatic activity of B16 melanoma cells. Clin Exp Metastasis. 1999;17(7):567–74.
Kurzer M, Meguid MM. Cancer and protein metabolism. Surg Clin North Am. 1986;66(5):969–1001.
Wiggins T, Kumar S, Markar SR, Antonowicz S, Hanna GB. Tyrosine, phenylalanine, and tryptophan in gastroesophageal malignancy: a systematic review. Cancer Epidemiol Biomarkers Prev. 2015;24(1):32–8.
Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361(9371):1810–2.
Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod. 2012;27(5):1327–31.
Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748–58.
Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Sign. 2018;29(17):1727–45.
Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721–33.
Akino N, Wada-Hiraike O, Terao H, Honjoh H, Isono W, Fu H, Hirano M, Miyamoto Y, Tanikawa M, Harada M, Hirata T, Hirota Y, Koga K, Oda K, Kawana K, Fujii T, Osuga Y. Activation of Nrf2 might reduce oxidative stress in human granulosa cells. Mol Cell Endocrinol. 2018;470:96–104.
Acknowledgements
This manuscript has been edited for English language and spelling by Enago.
Funding
This work was supported by the National Key R&D Program of China (No. 2017YFC1002003) and the Ministry of Science and Technology of China (No. 2017YFC1001601).
Author information
Authors and Affiliations
Contributions
R.C.C.: study design, manuscript writing, and critical discussion. B.T. and E.M.G.: study design, experiments execution, data analysis, manuscript writing, and critical discussion. F.Y., Y.B.L., Z.Y.Y, L.C.C, Y.J.X., W.M.H, L.W., and K.G.: experiments execution and manuscript comment. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Medical and Life Science Ethics Committee of Tongji University (approval No. 2017yxy001). All participants involved in this study have given their consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turathum, B., Gao, EM., Yang, F. et al. Role of pyroglutamic acid in cumulus cells of women with polycystic ovary syndrome. J Assist Reprod Genet 39, 2737–2746 (2022). https://doi.org/10.1007/s10815-022-02647-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02647-1