Abstract
Propose
Does an annotation-free embryo scoring system based on deep learning and time-lapse sequence images correlate with live birth (LB) and neonatal outcomes?
Methods
Patients who underwent SVBT cycles (3010 cycles, mean age: 39.3 ± 4.0). Scores were calculated using the iDAScore software module in the Vitrolife Technology Hub (Vitrolife, Gothenburg, Sweden). The correlation between iDAScore, LB rates, and total miscarriage (TM), including 1st- and 2nd-trimester miscarriage, was analysed using a trend test and multivariable logistic regression analysis. Furthermore, the correlation between the iDAScore and neonatal outcomes was analysed.
Results
LB rates decreased as iDAScore decreased (P < 0.05), and a similar inverse trend was observed for the TM rates. Additionally, multivariate logistic regression analysis showed that iDAScore significantly correlated with increased LB (adjusted odds ratio: 1.811, 95% CI: 1.666–1.976, P < 0.05) and decreased TM (adjusted odds ratio: 0.799, 95% CI: 0.706–0.905, P < 0.05). There was no significant correlation between iDAScore and neonatal outcomes, including congenital malformations, sex, gestational age, and birth weight. Multivariate logistic regression analysis, which included maternal and paternal age, maternal body mass index, parity, smoking, and presence or absence of caesarean section as confounding factors, revealed no significant difference in any neonatal characteristics.
Conclusion
Automatic embryo scoring using iDAScore correlates with decreased miscarriage and increased LB and has no correlation with neonatal outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Single vitrified-warmed blastocyst transfer (SVBT) is increasingly used as the main strategy for in vitro fertilisation (IVF) and embryo transfer treatment in many IVF laboratories [1,2,3]. SVBT enables the selection of the best blastocyst for transfer and promotes synchronisation and bidirectional communication between a receptive uterus and an implantation-competent blastocyst. Therefore, SVBT prevents multiple conceptions and improves implantation and pregnancy rates as well as neonatal outcomes.
Live birth (LB) is the most important outcome of an IVF treatment. The accurate prediction of LB can reduce the cost and time of IVF by preventing implantation failure and miscarriage. Therefore, assessing embryos by predicting LB is important. In regard to blastocyst assessment, morphological grading based on the Gardner criteria is the most commonly used assessment method [4]. Studies of LB prediction based on blastocyst morphology suggested that the trophectoderm (TE) morphology and blastocyst expansion score can predict live birth [5, 6]. Additionally, determining the inner cell mass (ICM) grade may reduce the risk of early pregnancy loss [7]. As a step further, assessment of embryos by morphokinetic parameters using a time-lapse incubation system was reported as a promising strategy for LB prediction [8,9,10]. However, blastocyst assessment by Gardner criteria as well as embryo assessment by morphokinetic parameters is subjective and it has been reported that assessments are affected by both intra- and inter-observer variation [11]. Therefore, existing embryo and blastocyst grading systems must be improved by incorporating more objective assessment and reproducible variables.
Recently, the benefit of artificial intelligence (AI) technology for diagnostic purposes in human ART has been shown, particularly for pregnancy prediction after embryo transfer [12,13,14,15]. The use of AI for LB prediction after blastocyst transfer has been extensively studied [12, 13, 16,17,18]. AI can overcome the issue of subjective assessment for selecting blastocysts for transfer. The iDAScore® v1.0 model (Vitrolife, Gothenburg, Sweden) was developed based on the IVY deep learning model [19], and has been integrated directly into a time-lapse system. Therefore, extra equipment or image processing in another computational platform is not required and the IVF laboratory has direct access to embryo scores through the installed software. Our previous study suggested that objective embryo assessment using iDAScore performs as good or even better than traditional embryo assessment or more annotation-dependent ranking tools in various maternal age groups [20]. Furthermore, iDAScore does not require any manual, user-dependent annotations, enabling consistent and objective assessment of blastocysts.
Previously published studies suggested that blastocyst quality, which was based on manual traditional assessment of developmental speed and blastocyst morphology, does influence neonatal outcomes [21,22,23,24,25]. On the other hand, the correlation between basic neonatal outcomes and scores that are calculated by any automatic, AI-based blastocyst scoring system has not been evaluated yet and this is also true for iDAScore. Therefore, the clinical application of iDAScore still requires studies that analyse its correlation with neonatal outcomes.
The objective of this retrospective large cohort study was to investigate whether iDAScore correlates with LB and neonatal outcomes after autologous SVBT.
Material and methods
Patients and study design
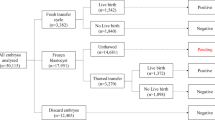
A total of 3010 patients undergoing 3010 autologous SVBT cycles (1 patient: 1 cycle) at the centre was included between January 2019 and May 2020. In this study, preimplantation genetic testing (PGT) was not involved. During the study period, only single-embryo transfers were performed in our centre, and an exclusive single-embryo transfer policy was strictly followed. No pre-implantation genetic testing was performed. LB and neonatal outcomes were ascertained for all patients using information from a written patient questionnaire and/or from the treating obstetrician. Patients without a complete time-lapse sequence, e.g. due to instrument maintenance, were excluded from the study. Furthermore, iDAScore can only be calculated for embryos that are cultured for at least 112 h after insemination. Therefore, cycles where this time period was not reached were excluded. SVBT was performed on days 4.5–5 after ovulation during a spontaneous natural cycle. The survival rate after thawing was 99.9% (3010/3012). The cycles were stratified into five maternal age groups described by the Society for Assisted Reproductive Technology (< 35, 35–37, 38–40, 41–42, and > 42 years old).
All transferred embryos were evaluated retrospectively using iDAScore v1.0 (Vitrolife) as an annotation-free scoring system based on deep learning. The iDAScore groups were divided into quartiles (1.0–7.2, 7.3–8.6, 8.7–9.2, and 9.3–9.9).
The main outcome measure was the LB rate (LB at ≥ 22 weeks of pregnancy) per embryo transfer procedure. Secondary outcomes were total miscarriage and basic neonatal outcomes, which were analysed for 752 singleton deliveries.
Minimal ovarian stimulation, oocyte retrieval, fertilisation procedures, and embryo culture
All patients underwent the minimal ovarian stimulation protocol described in previous studies [26]. Intracytoplasmic sperm injection (ICSI) was performed at 4 to 5 h after egg retrieval (ER). Following ICSI, oocytes were transferred to a pre-equilibrated EmbryoSlide (Vitrolife) and incubated in a time-lapse incubator (EmbryoScope+ or EmbryoScope Flex, Vitrolife). Embryo slides were prepared according to the manufacturer’s instructions. A one-step medium (NAKA Medical, Tokyo, Japan) was used for embryo culture. The culture dishes were covered with mineral oil (Ovoil, Vitrolife), and all embryos were cultured at 37 °C under a gas phase of 6% CO2, 5% O2, and 89% N2 from day 1 to day 5, 6, or 7.
Embryo observation, blastocyst monitoring, and vitrification
Normally fertilised zygotes with two pronuclei were cultured until the blastocyst stage. Embryos were observed using EmbryoViewer software without removing the culture dish from the incubator. For vitrification on day 5 or 6, blastocysts were required to attain an inner diameter of > 160 μm [27]. These blastocysts were vitrified immediately according to the Cryotop method [28]. If the developing embryo did not fulfil the criteria, it was cultured for a maximum of 7 days. For blastocyst vitrification on day 7, blastocysts were required to attain an inner diameter of > 180 μm [29]. If an embryo did not fulfil these criteria by day 7, it was discarded. The blastocyst inner diameter was measured using EmbryoViewer software.
Deep learning model (iDAScore)
The iDAScore v1.0 model [30] was developed using a 3D convolutional neural network and was trained on time-lapse sequence images [19]. The only input to the model was the images of a time-lapse sequence, and the output was a numerical score of 1–9.9, correlating with the likelihood of obtaining a positive foetal heartbeat. Therefore, iDAScore used no subjective human-annotated data for training, and thus no morphokinetic variables were required.
The training data for iDAScore included data from our clinic. However, all data in the current study was obtained after the data used for training. The transferred blastocysts were retrospectively scored using the model from the commercially available iDAScore v1.0.
Post-warming embryo culture and vitrified-warmed blastocyst transfer procedure
The embryo transfer procedure was performed as described previously [2]. In 2076 cycles only one vitrified blastocyst was available for SVBT. In 934 cycles, more than one blastocyst was available and blastocysts were selected for warming using our original grading system [29, 31] and applied prior to freezing. According to our previous study, assisted hatching using a laser (Saturn 5, CooperSurgical, USA) was performed after warming [32]. Surviving blastocysts were cultured for 30 min to 2 h until blastocoel re-expansion was confirmed. Only blastocysts in which the blastocoel size remained the same or increased relative to the size before vitrification were transferred, whereas degenerating blastocysts were discarded.
Luteal support was provided depending on the blood progesterone level on the day of embryo transfer. Patients with a serum progesterone level > 12 ng/mL on the day of embryo transfer were only administered dydrogesterone (30 mg/day orally; Daiichi Sankyo, Tokyo, Japan). SVBT was not performed in patients with serum progesterone levels of < 8 ng/mL. Patients whose progesterone levels were 8–12 ng/mL were administered progesterone intravaginally (Luteum Vaginal Suppository, ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) until the eighth week of pregnancy.
For clinical outcomes, we used the following definitions: chemical abortion, serum hCG level was over 20 IU/m: [33], but no gestational sac; clinical pregnancy, with a confirmed gestational sac at 6–7 weeks of pregnancy; ongoing pregnancy: a confirmed foetal heartbeat at 9 weeks of pregnancy; LB: LB at ≥ 22 weeks of pregnancy; 1st-trimester abortion: gestational sac was confirmed, but no foetal heartbeat; 2nd-trimester abortion: foetal heartbeat was confirmed, but no delivery; and total miscarriage (TM): gestational sac was confirmed, but no delivery.
During the first trimester, pregnancies were followed approximately until 9 weeks of ongoing gestation (confirmed foetal heartbeat), at which point patients were referred to their obstetrician for subsequent care.
Neonatal outcomes
Neonatal outcomes (excluding monozygotic twins) for gestational age, congenital malformation rate, male sex ratio, birth weight, rate of small for gestational age (SGA), and large for gestational age (LGA) were compared among the iDAScore groups. SGA and LGA in Japan are defined as a foetal growth curve below the 3rd and above the 97th percentile from the registry database of the Japan Society of Obstetrics and Gynaecology, respectively [34]. In addition, correlations between iDAScore and neonatal outcomes were analysed using regression analysis with maternal age, paternal age, maternal body mass index (BMI), parity, smoking, and with or without caesarean section as confounding factors.
Statistical analysis
A chi-square test with Bonferroni correction was used to compare categorical variables among groups. Nominal variables were analysed using the Cochran-Armitage test to detect trends, and Wilcoxon rank-sum test was used to compare continuous variables. The discrimination performance of the iDAScore model was evaluated using receiver operating characteristic analysis. Multivariate logistic regressions were used to analyse the relationship between iDAScore and LB or TM. Only factors with P < 0.1 in univariate logistic regression analysis were included in multivariate logistic regression to calculate the adjusted odds ratios (aORs). Multiple linear regression analysis was used to control impacts by gender and gestational age when analysing association between birth weight and iDAScore. The assumption that the residuals were normally distributed was tested using normal quantile plots. Additionally, Z scores were calculated using national birthweight reference [35]. Comparison of AUCs was performed using a paired two-tailed DeLong’s test. An AUC of 0.5 is equivalent to random classification, and 1.0 is equivalent to 100% correct classification. Statistical analyses were performed using JMP software (version 10.0; SAS Institute, Cary, NC, USA) and R (version 3.6.1, 2019–07-05).
Results
Table 1 shows the participant characteristics for each maternal age group. Paternal age, number of previous egg retrievals and embryo transfer, miscarriage history, and iDAScore significantly differed among the maternal age groups (P < 0.05).
Table 2 shows the clinical outcomes after SVBT stratified by iDAScore group. For βhCG positive, clinical pregnancy, 1st-trimester miscarriage, ongoing pregnancy, TM, and LB, there was a significant correlation with the iDAScore group (P < 0.05). However, no correlation was found between the iDAScore group and chemical abortion and 2nd-trimester miscarriage. Figure 1 shows box plots for the distribution of the iDAScore for LB-positive and LB-negative blastocysts within each age group. Within each maternal age group, iDAScore was significantly higher in the LB-positive group than in the negative group (P < 0.05). Furthermore, iDAScores in 42-year-old groups were significantly lower than in other age groups (P < 0.05).
LB and miscarriage rates on iDAScore stratified by maternal age
We analysed the correlation between LB rates and each iDAScore group stratified by maternal age. The LB rates were significantly different (P < 0.05) between groups and decreased progressively with decreasing scores for all maternal age groups (Fig. 2). Table 3 shows the results of univariate and multivariate logistic regression analyses for the LB probabilities. Maternal age, number of previous ER, and iDAScore were significantly correlated with a positive LB probability (maternal age: aOR 0.814, 95% confidence interval (CI) 0.789–0.839; no. of previous ER: aOR 0.963, 95% CI 0.925–0.999; iDAScore: aOR: 1.535, 95% CI: 1.358–1.736, P < 0.05). The ability of iDAScore to discriminate between positive and negative LB was estimated using the area under the receiver operating characteristic curve (AUC) metric. The AUC of all patients was 0.700. In the subgroups, the AUCs were as follows: < 35-year age group: 0.705, 35–37-year age group: 0.681, 38–40-year age group: 0.654, 41–42-year age group: 0.694 and ≥ 43-year age group: 0.771.
Furthermore, we analysed the correlation between TM and iDAScore groups within each maternal age group. The TM rates significantly increased progressively with decreasing iDAScores, except in the 38–40-year-old group and > 42-year-old group (Fig. 3). Table 4 shows the results of univariate and multivariate logistic regression analyses for TM. Maternal age and iDAScores significantly correlated with TM (maternal age: aOR 1.207, 95% CI 1.154–1.261; iDAScore: aOR: 0.817, 95% CI: 0.716–0.932, P < 0.05).
TM rates (%) on the y-axis and “Age group” on the x-axis. TM rates (%) in subgroups stratified by maternal age group and iDAScore group, stratified by quartiles. TM rates significantly increased progressively with decreasing iDAScores, except in the 38–40-year-old group and > 42-year-old group (P < 0.05). TM = total miscarriage
Neonatal outcomes
Table 5 shows a comparison of neonatal outcomes among the iDAScore groups after SVBT and singleton delivery. There were no significant differences in the congenital malformation rates, male sex ratio in neonates, rate of caesarean section, gestational age, early preterm birth, very early preterm birth, extremely early preterm birth, birth weight, and rates of LGA and SGA infants. Table 6 shows the unadjusted and aORs for low birth weight, SGA, LGA preterm birth, male sex, and major congenital malformations. Multivariate logistic regression analysis, which included maternal and paternal age, maternal body mass index, parity, smoking, and presence or absence of caesarean section as confounding factors, revealed no significant difference in any neonatal characteristics. Multiple linear regression analysis showed no association between iDAScore and birthweight (Table 7).
Discussion
Most of the current embryo selection models based on AI and time lapse have been developed and tested with foetal heartbeat as end point [36]. However, a more clinically relevant end point is live birth. Our study is to our knowledge the largest study where an embryo selection model is tested on SVBT using LB as end point. Few studies have investigated the correlation between embryo selection models and LB predictions [17, 37]. In addition, these models were developed on data from one clinic and, thus, testing of these models did use internal validation data. In contrast, this study can be considered as a temporal external validation as the data was obtained after the development of the model [38]. We identified a correlation between an automatic-embryo evaluation system based on AI (iDAScore) and LB and/or neonatal outcomes using an independent dataset. The ability of iDAScore to discriminate between positive and negative LB within different maternal age groups was lowest for the maternal age group 38–40 and increased toward both younger and older maternal ages. Figure 1 shows that the score distribution of the LB + embryos was quite similar for all age groups. However, mean scores for the LB − embryos were highest for the 38–40 age group and tended to be lower for the younger and older maternal age groups. Thus, it is hypothesised that the difference in discrimination performance is due to that the available useable blastocyst were more homogeneous for the 38–40 age group. For the other age groups, the quality of the available useable blastocysts was more heterogenous and thereby gave rise to a higher AUC due to the easier discrimination.
Currently, embryos are selected for transfer based on our in-house grading system, which grades embryos according to a combination of maternal age and morphology [31]. In order to compare LB prediction performance between iDAScore and our in-house grading system, we adjusted iDAScore for maternal age. The AUC for our in-house grading system was 0.757. For iDAScore, the age-adjusted AUC of 0.794 was significantly higher than in-house grading (Supplemental Table 1). However, it should be noted that when retrospectively comparing AI models against current embryo selection practice there will be an inevitable selection bias [35].
In addition, we found that iDAScore correlated with TM with a significant decrease in TM with increasing iDAScore. A previous study suggested that miscarriage could be predicted based on the trophectoderm grade and embryo morphokinetic [10, 39]. However, evaluation of trophectoderm and direct cleavage is subjective and affected by both inter- and intra-observer variations [40]. A potentially more consistent and generalizable estimation of TM can thus be obtained by using iDAScore. However, due to the nature of deep learning, we currently do not know which features the AI learned. It should be noted that iDAScore has been trained to predict foetal heartbeat (FHB), so the observed correlations with LB and TM must also be important for the likelihood of implantation. This could in example be the genetic or metabolomic competence of the embryo. Further studies are necessary to investigate which input features are important for the AI and miscarriage prediction.
iDAScore did not correlate with basic neonatal outcomes such as malformation, birth weight, gestational age, and male sex rates. Previous studies suggested that blastocyst morphology, including the inner cell mass and trophectoderm, and morphokinetics correlate with sex [21, 41], birthweight [42, 43], and congenital anomalies [44]. However, the correlation between blastocyst quality and neonatal outcomes remains controversial. Several of the studies suggested that blastocyst morphology was related to sex ratio and birthweight [21, 25]. Both studies relied on operator-dependent grading of blastocyst morphology at a single time-point. In contrast, our results were obtained by a completely objective assessment method using timelapse image sequences over the entire embryo development. This study showed no correlation to sex ratio and birthweight, although we observed a non-significant trend of increasing male sex ratio with increasing iDAScores. As pointed out by Afnan et al. [45], it is important to check for unwanted side effects introduced by embryo selection based on black-box AI models. Potential side effects can occur if traits are indirectly correlated with high likelihood of implantation. For example, embryo developmental speed, morphokinetic parameters, and blastocyst grade [21, 40, 46, 47] have been reported to correlate with both sex ratio and implantation likelihood. In a study on iDAScore, it was shown that scores correlate with both time to blastocyst and blastocyst grades [30]. This could possibly be one of the causes for the observed non-significant trend in the male sex ratio. However, such a bias in male sex ratio is also present in current selection methods based on Gardner grading [21, 47]. To get a better estimation of any potential bias in sex ratios, we suggest that AI models should be tested on PGT-A data. This will allow for an estimation of how frequent a male embryo is chosen over a female embryo in a specific cycle. In general, we suggest that black-box models should always be tested for potential unwanted biases and long-term effects.
Our results show that LB probabilities significantly correlate with iDAScore, number of previous ER, day of blastocyst vitrification (day 5 to day 6), and maternal age. Thus, the inclusion of age as input to iDAScore will inevitably improve the overall ranking performance (i.e. the AUC). However, this represents an overoptimistic estimate of ranking performance as age does not improve sorting capability on treatment level where age is constant. Hence, when age is used as model input [16], it is important to test the ranking performance on age sub-groups to get a better understanding of the performance on treatment level. If the aim of an AI model or other blastocyst assessment model are to predict the actual implantation potential in a clinic, it is necessary to make a clinic-specific calibration of the model output using maternal age and other clinic specific covariates [13]. As shown in our previous study, it is possible to make a clinic-specific calibration of KIDScore D5 to reflect the actual chance of an FHB or LB within different age groups [9]. This can be used for patient counselling on whether to transfer a low score blastocyst. In addition, a calibrated model will also allow for a clinic-specific threshold to be used for deciding whether a given blastocyst should be vitrified. It should be noted that a clinic-specific calibration will not change the ranking and transfer order within a single cycle. The number of previous ER correlated with the LB probability. This was probably due to the poor prognosis for patients with many previous ER. The day of blastocyst vitrification also correlated with the LB probability. This suggests that for the prediction of the actual LB probability, iDAScore needs to be calibrated differently for day 5 and day 6. However, a previous study in our clinic suggested that day of blastocyst vitrification did not influence the FHB prediction ability of iDAScore [20]. Therefore, before using iDAScore for LB prediction, clinics should verify if the model should be calibrated differently for days 5 and 6.
The iDAScore model was developed based on the IVY model [19]. The performance of the IVY model was evaluated on a significantly different sub-set of embryos as their test data set regarded discarded embryos as FHB − . Thus, their reported high AUC of 0.93 is a measure of the discrimination between FHB + vs (FHB − and discarded embryos). This is different from the current study where the test data set only includes transferred embryos and specifically discarded embryos are not included in the AUC calculation. In the analysis of iDAScore v1.0 by Berntsen et al. [30], the AUC for FHB + vs (FHB − and discarded) was 0.95 and for FHB + vs FHB − the AUC was 0.67. The differences between these two AUC values clearly show that it is much easier to discriminate between FHB + and discarded embryos than to discriminate among transferred embryos alone. However, from a usability point of view, it is important that an embryo selection model can identify the discarded embryos so that the model can be used on all embryos without any preselection by the user. As described in Berntsen et al. [30], this can be achieved by including discarded embryos in the training data. However, from a clinical perspective, we believe that the most important performance measure is the discriminative performance on a test set with only transferred embryos.
The iDAScore does not always correlate with the results of blastocyst assessment by other models e.g. Gardner criteria or KIDScore D5 [20]. This is one of the issues in embryo assessment by artificial intelligence. To address this issue, the final decision should be done by humans who are medical doctors or embryologists. In this regard, the medical staff who selects embryos for transfer needs to deeply understand embryo assessments models including traditional models. Furthermore, for the time being we recommend that blastocysts should be assessed by morphology or morphokinetics along with AI models. Such data will be helpful in the final decision which blastocyst to select for transfer and in patients counselling, especially when embryo selection by artificial intelligence scoring system is uninterpretable.
A major limitation of our study is that it was based on minimal stimulation and natural cycle IVF treatment, involving only insemination by ICSI and a freeze-all strategy whereby all transferred blastocysts had previously been vitrified. The vitrification procedure may cause a partial damage to blastocysts, thereby decreasing the overall implantation potential. Therefore, the ability of iDAScore to predict pregnancy outcomes may differ between fresh and frozen blastocyst transfers. Consequently, studies of fresh blastocyst transfers are required to determine the efficacy of the iDAScore in regard to TM, LB, and neonatal outcome. The iDAScore model was developed for FHB + prediction using data from 18 fertility centres that use different IVF procedures [30]; however, most of the data were from cycles with a fresh embryo transfer. Further studies performed in other fertility centres with different settings and practices are required to support the general applicability of this approach. As only minimal stimulation was used, studies using standard controlled ovarian stimulation are required to analyse the efficacy of selection in elective blastocyst transfer situations, preferably by using a randomised controlled trial design. Additionally, iDAScore can only be used in a time-lapse system (EmbryoScope+) equipped with the adequate software (Vitrolife Technology Hub). In addition, this study was retrospective in nature and thus may have limitations. Therefore, in future studies, randomised controlled trials are required.
Conclusions
Objective embryo assessment using a completely automatic and annotation-free model, iDAScore, is correlated with LB and TM. Furthermore, iDAScore was not correlated with any neonatal outcome parameters. iDAScore does not require any manual, user-dependent annotations, enabling objective assessment of embryos that are cultured to the blastocyst stage. Therefore, iDAScore is an optimal method for scoring embryos and prioritising blastocysts for transfer without compromising live birth and neonatal outcomes.
Change history
31 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10815-022-02605-x
References
Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, Ata B, Cohen J, Munne S. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril. 2017;107:723–30.
Kato K, Ezoe K, Yabuuchi A, Fukuda J, Kuroda T, Ueno S, Fujita H, Kobayashi T. Comparison of pregnancy outcomes following fresh and electively frozen single blastocyst transfer in natural cycle and clomiphene-stimulated IVF cycles. Hum Reprod Open. 2018;3:hoy006.
Wei D, Liu J-Y, Sun Y, Shi Y, Zhang B, Liu J-Q, Tan J, Liang X, Cao Y, Wang Z, Qin Y, Zhao H, Zhou Y, Ren H, Hao G, Ling X, Zhao J, Zhang Y, Qi X, Zhang L, Deng X, Chen X, Zhu Y, Wang X, Tian L-F, Lv Q, Ma X, Zhang H, Legro RS, Chen Z-J. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393:1310–8.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8.
Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289–96.
Thompson SM, Onwubalili N, Brown K, Jindal SK, McGovern PG. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET): a national study. J Assist Reprod Genet. 2013;30:1577–81.
Van den Abbeel E, Balaban B, Ziebe S, Lundin K, Cuesta MJ, Klein BM, Helmgaard L, Arce JC. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod Biomed Online. 2013;27:353–61.
Fishel S, Campbell A, Montgomery S, Smith R, Nice L, Duffy S, Jenner L, Berrisford K, Kellam L, Smith R, Foad F, Beccles A. Time-lapse imaging algorithms rank human preimplantation embryos according to the probability of live birth. Reprod Biomed Online. 2018;37:304–13.
Kato K, Ueno S, Berntsen J, Ito M, Shimazaki K, Uchiyama K, Okimura T. Comparing prediction of ongoing pregnancy and live birth outcomes in patients with advanced and younger maternal age patients using KIDScore day 5: a large-cohort retrospective study with single vitrified-warmed blastocyst transfer. Reprod Biol Endocrinol. 2021;19:98.
Pribenszky C, Nilselid AM, Montag M. Time-lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: a meta-analysis. Reprod Biomed Online. 2017;35:511–20.
Storr A, Venetis CA, Cooke S, Kilani S, Ledger W. Inter-observer and intra-observer agreement between embryologists during selection of a single Day 5 embryo for transfer: a multicenter study. Hum Reprod. 2017;32:307–14.
Bori L, Dominguez F, Fernandez EI, Del Gallego R, Alegre L, Hickman C, Quiñonero A, Nogueira MFG, Rocha JC, Meseguer M. An artificial intelligence model based on the proteomic profile of euploid embryos and blastocyst morphology: a preliminary study. Reprod Biomed Online. 2020;42:340–50.
Bormann CL, Kanakasabapathy MK, Thirumalaraju P, Gupta R, Pooniwala R, Kandula H, Hariton E, Souter I, Dimitriadis I, Ramirez LB, Curchoe CL, Swain J, Boehnlein LM, Shafiee H. Performance of a deep learning based neural network in the selection of human blastocysts for implantation. Elife. 2020; 9.
Swain J, VerMilyea MT, Meseguer M, Ezcurra D, Fertility AIFG. AI in the treatment of fertility: key considerations. J Assist Reprod Genet. 2020;37:2817–24.
Zaninovic N, Rosenwaks Z. Artificial intelligence in human in vitro fertilization and embryology. Fertil Steril. 2020;114:914–20.
Khosravi P, Kazemi E, Zhan Q, Malmsten JE, Toschi M, Zisimopoulos P, Sigaras A, Lavery S, Cooper LAD, Hickman C, Meseguer M, Rosenwaks Z, Elemento O, Zaninovic N, Hajirasouliha I. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. npj Digit Med. 2019;21.
Miyagi Y, Habara T, Hirata R, Hayashi N. Feasibility of deep learning for predicting live birth from a blastocyst image in patients classified by age. Reprod Med Biol. 2019;18:190–203.
VerMilyea M, Hall JMM, Diakiw SM, Johnston A, Nguyen T, Perugini D, Miller A, Picou A, Murphy AP, Perugini M. Development of an artificial intelligence-based assessment model for prediction of embryo viability using static images captured by optical light microscopy during IVF. Hum Reprod. 2020;35:770–84.
Tran D, Cooke S, Illingworth PJ, Gardner DK. Deep learning as a predictive tool for fetal heart pregnancy following time-lapse incubation and blastocyst transfer. Hum Reprod. 2019;34:1011–8.
Ueno S, Berntsen J, Ito M, Uchiyama K, Okimura T, Yabuuchi A, Kato K. Pregnancy prediction performance of an annotation-free embryo scoring system on the basis of deep learning after single vitrified-warmed blastocyst transfer: a single-center large cohort retrospective study. Fertil Steril. 2021;116:1172–80.
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, Katz-Jaffe MG, Wells D. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–4.
Ebner T, Tritscher K, Mayer RB, Oppelt P, Duba HC, Maurer M, Schappacher-Tilp G, Petek E, Shebl O. Quantitative and qualitative trophectoderm grading allows for prediction of live birth and gender. J Assist Reprod Genet. 2016;33:49–57.
Li M, Yin M, Wu L, Yan Z, Lyu Q, Yan Z, Li B. Pregnancy and neonatal outcomes of morphologically grade CC blastocysts: are they of clinical value? Arch Gynecol Obstet. 2020;302:1511–21.
Park DS, Kim JW, Chang EM, Lee WS, Yoon TK, Lyu SW. Obstetric, neonatal, and clinical outcomes of day 6 vs. day 5 vitrified-warmed blastocyst transfers: retrospective cohort study with propensity score matching. Front Endocrinol (Lausanne). 2020;11:499.
Zhang J, Huang J, Liu H, Wang B, Yang X, Shen X, Mao X, Wang Y, Kuang Y. The impact of embryo quality on singleton birthweight in vitrified-thawed single blastocyst transfer cycles. Hum Reprod. 2020;35:308–16.
Kato K, Takehara Y, Segawa T, Kawachiya S, Okuno T, Kobayashi T, Bodri D, Kato O. Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-center Japanese cohort. Reprod Biol Endocrinol. 2012;10:35.
Okimura T, Kuwayama M, Segawa T, Takehara Y, Kato K, Kato O. Relations between the timing of transfer, expansion size and implantation ratesin frozen thawed single blastocyst transfer. Fertil Steril. 2009;92:S246.
Mori C, Yabuuchi A, Ezoe K, Murata N, Takayama Y, Okimura T, Uchiyama K, Takakura K, Abe H, Wada K, Okuno T, Kobayashi T, Kato K. Hydroxypropyl cellulose as an option for supplementation of cryoprotectant solutions for embryo vitrification in human assisted reproductive technologies. Reprod Biomed Online. 2015;30:613–21.
Ueno S, Uchiyama K, Kuroda T, Okimura T, Yabuuchi A, Kobayashi T, Kato K. Establishment of day 7 blastocyst freezing criteria using blastocyst diameter for single vitrified-warmed blastocyst transfer from live birth outcomes: a single-center, large cohort, retrospectively matched study. J Assist Reprod Genet. 2020;37:2327–35.
Berntsen J, Rimestad J, Lassen JT, Tran D, Kragh MF. Robust and generalizable embryo selection based on artificial intelligence and time-lapse image sequences. PLoS ONE. 2022;17(2):e0262661.
Kato K, Ueno S, Yabuuchi A, Uchiyama K, Okuno T, Kobayashi T, Segawa T, Teramoto S. Women’s age and embryo developmental speed accurately predict clinical pregnancy after single vitrified-warmed blastocyst transfer. Reprod Biomed Online. 2014;29:411–6.
Ueno S, Ezoe K, Yabuuchi A, Uchiyama K, Okimura T, Okuno T, Kobayashi T, Kato K. Complete zona pellucida removal from vitrified-warmed human blastocysts facilitates earlier in-vitro attachment and outgrowth. Reprod Biomed Online. 2016;33:140–8.
Ueno S, Ezoe K, Abe T, Yabuuchi A, Uchiyama K, Okuno T, Kobayashi T, Kato K. Maternal age and initial beta-hCG levels predict pregnancy outcome after single vitrified-warmed blastocyst transfer. J Assist Reprod Genet. 2014;31:1175–81.
Itabashi K, Miura F, Uehara R, Nakamura Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr Int. 2014;56:702–8.
Minisry of Health, Labour and Welfare, Child and Family Policy Bureau, General Affairs Division. Japan National growth survey on preschool children. 2012.
Kragh MF, Karstoft H. Embryo selection with artificial intelligence: how to evaluate and compare methods? J Assist Reprod Genet. 2021;38:1675–89.
Sawada Y, Sato T, Nagaya M, Saito C, Yoshihara H, Banno C, Matsumoto Y, Matsuda Y, Yoshikai K, Sawada T, Ukita N, Sugiura-Ogasawara M. Evaluation of artificial intelligence using time-lapse images of IVF embryos to predict live birth. Reprod Biomed Online. 2021;43:843–52.
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1-73.
Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, Endo T, Saito T, Asada Y. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Steril. 2012;98:361–7.
Cimadomo D, Sosa Fernandez L, Soscia D, Fabozzi G, Benini F, Cesana A, Dal Canto MB, Maggiulli R, Muzzì S, Scarica C, Rienzi L, De Santis L. Inter-centre reliability in embryo grading across several IVF clinics is limited: implications for embryo selection. Reprod Biomed Online. 2022;44:39–48.
Bronet F, Nogales MC, Martinez E, Ariza M, Rubio C, Garcia-Velasco JA, Meseguer M. Is there a relationship between time-lapse parameters and embryo sex? Fertil Steril. 2015;103:396–401.
Licciardi F, McCaffrey C, Oh C, Schmidt-Sarosi C, McCulloh DH. Birth weight is associated with inner cell mass grade of blastocysts. Fertil Steril. 2015;103:382–7.
Xie Q, Du T, Zhao M, Gao C, Lyu Q, Suo L, Kuang Y. Advanced trophectoderm quality increases the risk of a large for gestational age baby in single frozen-thawed blastocyst transfer cycles. Hum Reprod. 2021;36:2111–20.
Doron-Lalehzari A, Wainstock T, Szaingurten-Solodkin I, Richter D, Zeadna A, Harlev A, Lunenfeld E, Levitas E, Har-Vardi I. Are morphokinetic parameters of embryo development associated with adverse perinatal outcomes following fresh blastocyst transfer? Reprod Biomed Online. 2021;42:207–16.
Afnan MAM, Liu Y, Conitzer V, Rudin C, Mishra A, Savulescu J, Afnan M. Interpretable, not black-box, artificial intelligence should be used for embryo selection. Hum Reprod Open. 2021;4:hoab040.
Huang B, Ren X, Zhu L, Wu L, Tan H, Guo N, Wei Y, Hu J, Liu Q, Chen W, Liu J, Li D, Liao S, Jin L. Is differences in embryo morphokinetic development significantly associated with human embryo sex? Biol Reprod. 2019;100:618–23.
Borgstrøm MB, Kesmodel US, Klausen TW, Danielsen AK, Thomsen T, Gabrielsen A, Englund ALM, Zedeler A, Povlsen BB, Troest B, Almind GJ, Fedder J, Kirk J, Hindkjær J, Lemmen JG, Petersen K, Haahr K, Petersen MR, Laursen S, Knudsen UB, Bentin-Ley U, Larsen T, Grøndahl MI. Developmental stage and morphology of the competent blastocyst are associated with sex of the child but not with other obstetric outcomes: a multicenter cohort study. Hum Reprod. 2021;37:119–28.
Acknowledgements
The authors wish to thank Markus Montag, PhD (ilabcomm GmbH), for his help in editing the initial manuscript draft. Also, the authors thank the clinical embryologists for their efforts in the laboratory and assistance with this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was reviewed and approved by the independent Institutional Review Board (approval number: 18–19, approved March 13, 2017).
Conflict of interest
J.B. is an employee and shareholder of Vitrolife and has a patent pending for Methods and Apparatus for Assessing Embryo Development and a patent issued for Embryo Quality Assessment Based on Blastomere Division and Movement.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to Retrospective Open Access.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ueno, S., Berntsen, J., Ito, M. et al. Correlation between an annotation-free embryo scoring system based on deep learning and live birth/neonatal outcomes after single vitrified-warmed blastocyst transfer: a single-centre, large-cohort retrospective study. J Assist Reprod Genet 39, 2089–2099 (2022). https://doi.org/10.1007/s10815-022-02562-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02562-5