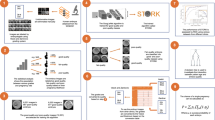
Abstract
Purpose
A deep learning artificial intelligence (AI) algorithm has been demonstrated to outperform embryologists in identifying euploid embryos destined to implant with an accuracy of 75.3% (1). Our aim was to evaluate the performance of highly trained embryologists in selecting top quality day 5 euploid blastocysts with and without the aid of a deep learning algorithm.
Materials and methods
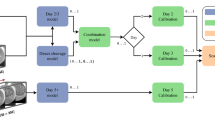
A non-overlapping series of 200 sets of day 5 euploid embryo images with known implantation outcomes was distributed to 17 highly trained embryologists. One embryo in each set was known to have implanted and one failed implantation. They were asked to select which embryo to transfer from each set. The same 200 sets of embryos, with indication of which embryo in each set had been identified by the algorithm as more likely to implant was then distributed. Chi-squared, t-test, and receiver operating curves were performed to compare the embryologist performeance with and without AI.
Results
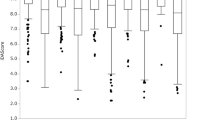
Fourteen embryologists completed both assessments. Embryologists provided with AI results selected successfully implanted embryos in 73.6% of cases compared to 65.5% for those selected using visual assessments alone (p < 0.001). All embryologists improved in their ability to select embryos with the aid of the AI algorithm with a mean percent improvement of 11.1% (range 1.4% to 15.5%). There were no differences in degree of improvement by embryologist level of experience (junior, intermediate, senior).
Conclusions
The incorporation of an AI framework for blastocyst selection enhanced the performance of trained embryologists in identifying PGT-A euploid embryos destined to implant.




Similar content being viewed by others
References
Strauss JF, Barbieri RL, editors. Yen & Jaffe’s reproductive endocrinology: physiology, pathophysiology, and clinical management. 8th ed. Philadelphia, PA: Elsevier; 2019.
Bromer JG, Seli E. Assessment of embryo viability in assisted reproductive technology: shortcomings of current approaches and the emerging role of metabolomics. Curr Opin Obstet Gynecol. 2008;20(3):234–41. https://doi.org/10.1097/GCO.0b013e3282fe723d.
Munné S, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071-1079.e7. https://doi.org/10.1016/j.fertnstert.2019.07.1346.
Irani M, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107(3):664–70. https://doi.org/10.1016/j.fertnstert.2016.11.012.
Baxter Bendus AE, Mayer JF, Shipley SK, Catherino WH. Interobserver and intraobserver variation in day 3 embryo grading. Fertil Steril. 2006;86(6):1608–15. https://doi.org/10.1016/j.fertnstert.2006.05.037.
Bormann CL, et al. Consistency and objectivity of automated embryo assessments using deep neural networks. Fertil Steril. 2020;113(4):781-787.e1. https://doi.org/10.1016/j.fertnstert.2019.12.004.
Bormann CL, et al. Performance of a deep learning based neural network in the selection of human blastocysts for implantation. eLife. 2020;9:e55301. https://doi.org/10.7554/eLife.55301.
Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81(3):551–5. https://doi.org/10.1016/j.fertnstert.2003.07.023.
Kanakasabapathy M, et al. An inexpensive, automated artificial intelligence (AI) system for human embryo morphology evaluation and transfer selection. Fertil Steril. 2019;111(4): e11. https://doi.org/10.1016/j.fertnstert.2019.02.047.
Thirumalaraju P, et al. Deep learning-enabled blastocyst prediction system for cleavage stage embryo selection. Fertil Steril. 2019;111(4): e29. https://doi.org/10.1016/j.fertnstert.2019.02.077.
Chavez-Badiola A, Flores-Saiffe-Farías A, Mendizabal-Ruiz G, Drakeley AJ, Cohen J. Embryo Ranking Intelligent Classification Algorithm (ERICA): artificial intelligence clinical assistant predicting embryo ploidy and implantation. Reprod Biomed Online. 2020;41(4):585–93. https://doi.org/10.1016/j.rbmo.2020.07.003.
Chen T-J, Zheng W-L, Liu C-H, Huang I, Lai H-H, Liu M. Using Deep Learning with Large Dataset of Microscope Images to Develop an Automated Embryo Grading System. Fertil Reprod. 2019;01(01):51–6. https://doi.org/10.1142/S2661318219500051.
Curchoe CL. “The paper chase and the big data arms race,”. J Assist Reprod Genet. 2021. p. s10815–021–02122–3. https://doi.org/10.1007/s10815-021-02122-3.
Curchoe CL, Flores-Saiffe Farias A, Mendizabal-Ruiz G, Chavez-Badiola A. Evaluating predictive models in reproductive medicine. Fertil Steril. 2020;114(5):921–6. https://doi.org/10.1016/j.fertnstert.2020.09.159.
Shear MA, et al. Blasts from the past: is morphology useful in PGT-A tested and untested frozen embryo transfers? Reprod Biomed Online. 2020;41(6):981–9. https://doi.org/10.1016/j.rbmo.2020.07.014.
Storr A, Venetis CA, Cooke S, Kilani S, Ledger W. Inter-observer and intra-observer agreement between embryologists during selection of a single Day 5 embryo for transfer: a multicenter study. Hum Reprod. 2017;32(2):307–14. https://doi.org/10.1093/humrep/dew330.
Paulson RJ. Preimplantation genetic screening: what is the clinical efficiency? Fertil Steril. 2017;108(2):228–30. https://doi.org/10.1016/j.fertnstert.2017.06.023.
Tiegs AW, et al. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing–based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2021;115(3):627–37. https://doi.org/10.1016/j.fertnstert.2020.07.052.
Funding
This work was partially supported by the Brigham Precision Medicine Developmental Award (Brigham Precision Medicine Program, Brigham and Women’s Hospital), Partners Innovation Discovery Grant (Partners Healthcare), and R01AI118502, and R01AI138800.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by CB, KJ, and VWF. The fist draft of the manuscript was written by VWF, and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Approval for this study was obtained from the ethics committee of Massachusetts General Hospital (IRB #2017P001339 and #2019P002392).
Conflict of interest
CB, HS, MK, and PT all have a patent WO2019068073A1 pending in relation to this work. Other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fitz, V.W., Kanakasabapathy, M.K., Thirumalaraju, P. et al. Should there be an “AI” in TEAM? Embryologists selection of high implantation potential embryos improves with the aid of an artificial intelligence algorithm. J Assist Reprod Genet 38, 2663–2670 (2021). https://doi.org/10.1007/s10815-021-02318-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02318-7