Abstract
Purpose
The aim of this study was to examine the ability and safety of papaverine supplementation for in vitro sperm motility enhancement. In addition, sperm motility enhancement of papaverine was compared to pentoxifylline and theophylline. The post-thaw spermatozoa were used as an asthenozoospermia model.
Methods
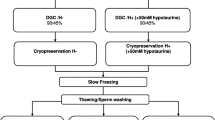
Post thaw sperm suspensions were divided into two groups: papaverine (100 μmol/L) and control, and each was investigated in two subgroups of 30- and 60-min exposure times. Detailed motility parameters were detected using a computerized sperm motility analyzer. Acrosomal status, viability, apoptosis, and DNA fragmentation were evaluated by flow cytometry. Furthermore, the motility-enhancing capacity of papaverine, pentoxifylline, and theophylline was compared.
Results
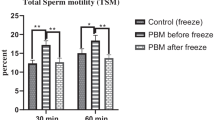
Cryopreservation impaired sperm parameters dramatically but no significant changes occurred in acrosomal status and apoptosis. Supplementation of papaverine enhanced motility parameters consistently at all exposure intervals, significantly. However, viability was lower at the 60th minute compared to the 30th minute (p=0.019). Papaverine did not alter any acrosomal or apoptotic markers at any time points. All of the compounds compared in this study increased the motility parameters, where theophylline supplementation provided significantly better improvement in total motility compared to papaverine and pentoxifylline.
Conclusion
Our results suggest that in vitro papaverine treatment for 30 min adequately improves motility of post-thaw sperm, without leading to acrosome reaction, DNA damage, and viability loss. Theophylline’s potency on increasing the ratio of total motile spermatozoa was found significantly superior than the two tested compounds. Prospective clinical studies with embryo production, pregnancy, and live birth data should be undertaken.






Similar content being viewed by others
Data Availability
Data obtained in this manuscript is available
References
Ebner T, Shebl O, Mayer RB, Moser M, Costamoling W, Oppelt P. Healthy live birth using theophylline in a case of retrograde ejaculation and absolute asthenozoospermia. Fertil Steril. 2014;101(2):340–3. https://doi.org/10.1016/j.fertnstert.2013.10.006.
Ebner T, Tews G, Mayer RB, Ziehr S, Arzt W, Costamoling W, et al. Pharmacological stimulation of sperm motility in frozen and thawed testicular sperm using the dimethylxanthine theophylline. Fertil Steril. 2011;96(6):1331–6. https://doi.org/10.1016/j.fertnstert.2011.08.041.
Yovich JM, Edirisinghe WR, Cummins JM, Yovich JL. Influence of pentoxifylline in severe male factor infertility. Fertil Steril. 1990;53(4):715–22.
Matson PL, Yovich JM, Edirisinghe WR, Junk SM, Yovich JL. An argument for the past and continued use of pentoxifylline in assisted reproductive technology. Hum Reprod. 1995;10(Suppl 1):67–71. https://doi.org/10.1093/humrep/10.suppl_1.67.
Negri P, Grechi E, Tomasi A, Fabbri E, Capuzzo A. Effectiveness of pentoxifylline in semen preparation for intrauterine insemination. Hum Reprod. 1996;11(6):1236–9. https://doi.org/10.1093/oxfordjournals.humrep.a019363.
Aribarg A, Sukcharoen N, Jetsawangsri U, Chanprasit Y, Ngeamvijawat J. Effects of pentoxifylline on sperm motility characteristics and motility longevity of postthaw cryopreserved semen using computer-assisted semen analysis (CASA). J Med Assoc Thail. 1994;77(2):71–5.
Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J Androl. 2006;27(1):45–52. https://doi.org/10.2164/jandrol.05079.
Yildirim G, Ficicioglu C, Akcin O, Attar R, Tecellioglu N, Yencilek F. Can pentoxifylline improve the sperm motion and ICSI success in the primary ciliary dyskinesia? Arch Gynecol Obstet. 2009;279(2):213–5. https://doi.org/10.1007/s00404-008-0671-y.
Nassar A, Mahony M, Morshedi M, Lin MH, Srisombut C, Oehninger S. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil Steril. 1999;71(5):919–23.
Stanic P, Sonicki Z, Suchanek E. Effect of pentoxifylline on motility and membrane integrity of cryopreserved human spermatozoa. Int J Androl. 2002;25(3):186–90.
Nabi A, Khalili MA, Fesahat F, Talebi A, Ghasemi-Esmailabad S. Pentoxifylline increase sperm motility in devitrified spermatozoa from asthenozoospermic patient without damage chromatin and DNA integrity. Cryobiology. 2017;76:59–64. https://doi.org/10.1016/j.cryobiol.2017.04.008.
Grassi G, Cappello N, Gheorghe MF, Salton L, Di Bisceglie C, Manieri C, et al. Exogenous platelet-activating factor improves the motility of human spermatozoa evaluated with C.A.S.A.: optimal concentration and incubation time. J Endocrinol Investig. 2010;33(10):684–90. https://doi.org/10.1007/bf03346670.
Barakat IA, Danfour MA, Galewan FA, Dkhil MA. Effect of various concentrations of caffeine, pentoxifylline, and kallikrein on hyperactivation of frozen bovine semen. Biomed Res Int. 2015;2015:948575–7. https://doi.org/10.1155/2015/948575.
Rees JM, Ford WC, Hull MG. Effect of caffeine and of pentoxifylline on the motility and metabolism of human spermatozoa. J Reprod Fertil. 1990;90(1):147–56. https://doi.org/10.1530/jrf.0.0900147.
Ortgies F, Klewitz J, Gorgens A, Martinsson G, Sieme H. Effect of procaine, pentoxifylline and trolox on capacitation and hyperactivation of stallion spermatozoa. Andrologia. 2012;44(Suppl 1):130–8. https://doi.org/10.1111/j.1439-0272.2010.01150.x.
Nekoonam S, Nashtaei MS. naji M, Zangi BM, Amidi F. Effect of Trolox on sperm quality in normozospermia and oligozospermia during cryopreservation. Cryobiology. 2016;72(2):106–11. https://doi.org/10.1016/j.cryobiol.2016.02.008.
Ho HC, Suarez SS. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction. 2001;122(4):519–26.
Glenn DR, McVicar CM, McClure N, Lewis SE. Sildenafil citrate improves sperm motility but causes a premature acrosome reaction in vitro. Fertil Steril. 2007;87(5):1064–70. https://doi.org/10.1016/j.fertnstert.2006.11.017.
Tournaye H, Van der Linden M, Van den Abbeel E, Devroey P, Van Steirteghem A. Effects of pentoxifylline on in-vitro development of preimplantation mouse embryos. Hum Reprod. 1993;8(9):1475–80.
Centola GM, Cartie RJ, Cox C. Differential responses of human sperm to varying concentrations of pentoxyfylline with demonstration of toxicity. J Androl. 1995;16(2):136–42.
Robaire B, Hinton B, Orgebin-Crist M. Knobil and Neill’s physiology of reproduction. In: Neill JD, editor. Physiology of Reproduction. 3rd ed. New York: Elsevier; 2006.
Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289(5479):625–8. https://doi.org/10.1126/science.289.5479.625.
Drobnis EZ, Nangia AK. Phosphodiesterase inhibitors (PDE inhibitors) and male reproduction. Adv Exp Med Biol. 2017;1034:29–38. https://doi.org/10.1007/978-3-319-69535-8_5.
Ain R, Uma Devi K, Shivaji S, Seshagiri PB. Pentoxifylline-stimulated capacitation and acrosome reaction in hamster spermatozoa: involvement of intracellular signalling molecules. Mol Hum Reprod. 1999;5(7):618–26. https://doi.org/10.1093/molehr/5.7.618.
Bergeron A, Hebert A, Guillemette C, Laroche A, Poulin MP, Aragon JP, et al. Papaverine-sensitive phosphodiesterase activity is measured in bovine spermatozoa. Andrology. 2017;5(1):169–79. https://doi.org/10.1111/andr.12290.
Marechal L, Guillemette C, Goupil S, Blondin P, Leclerc P, Richard FJ. Cyclic nucleotide phosphodiesterases in human spermatozoa and seminal fluid: presence of an active PDE10A in human spermatozoa. Biochim Biophys Acta. 2017;1861(2):147–56. https://doi.org/10.1016/j.bbagen.2016.11.006.
Mooradian AD, Morley JE, Kaiser FE, Davis SS, Viosca SP, Korenman SC. Biweekly intracavernous administration of papaverine for erectile dysfunction. W J Med. 1989;151(5):515–7.
Tardif S, Madamidola OA, Brown SG, Frame L, Lefièvre L, Wyatt PG, et al. Clinically relevant enhancement of human sperm motility using compounds with reported phosphodiesterase inhibitor activity. Hum Reprod. 2014;29(10):2123–35. https://doi.org/10.1093/humrep/deu196.
Hamada A, Wasik M, Gupta S, Agarwal A. Sperm banking: indications and regulations. 2014.
Ozkavukcu S, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet. 2008;25(8):403–11. https://doi.org/10.1007/s10815-008-9232-3.
Donovan A, Hanrahan JP, Kummen E, Duffy P, Boland MP. Fertility in the ewe following cervical insemination with fresh or frozen-thawed semen at a natural or synchronised oestrus. Anim Reprod Sci. 2004;84(3-4):359–68. https://doi.org/10.1016/j.anireprosci.2003.12.014.
Agnihotri SK, Agrawal AK, Hakim BA, Vishwakarma AL, Narender T, Sachan R, et al. Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell Dev Biol Anim. 2016;52(9):953–60. https://doi.org/10.1007/s11626-016-0061-x.
WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed; 2010.
Punyatanasakchai P, Sophonsritsuk A, Weerakiet S, Wansumrit S, Chompurat D. Comparison of cryopreserved human sperm in vapor and liquid phases of liquid nitrogen: effect on motility parameters, morphology, and sperm function. Fertil Steril. 2008;90(5):1978–82. https://doi.org/10.1016/j.fertnstert.2007.09.066.
Ormerod MG. The study of apoptotic cells by flow cytometry. Leukemia. 1998;12(7):1013–25. https://doi.org/10.1038/sj.leu.2401061.
Lewis SE, McKinney KA, Thompson W. Influence of pentoxifylline on human sperm motility in asthenozoospermic individuals using computer-assisted analysis. Arch Androl. 1994;32(3):175–83.
Terriou P, Hans E, Cortvrindt R, Avon C, Charles O, Salzmann J, et al. Papaverine as a replacement for pentoxifylline to select thawed testicular or epididymal spermatozoa before ICSI. Gynecol Obstet Fertil. 2015;43(12):786–90. https://doi.org/10.1016/j.gyobfe.2015.10.007.
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8.
Nagy Z, Liu J, Cecile J, Silber S, Devroey P, Van Steirteghem A. Using ejaculated, fresh, and frozen-thawed epididymal and testicular spermatozoa gives rise to comparable results after intracytoplasmic sperm injection. Fertil Steril. 1995;63(4):808–15.
Imoedemhe DA, Sigue AB, Pacpaco EL, Olazo AB. The effect of caffeine on the ability of spermatozoa to fertilize mature human oocytes. J Assist Reprod Genet. 1992;9(2):155–60.
Maxwell WM, Robinson SJ, Roca J, Molinia FC, Sanchez-Partida LG, Evans G. Motility, acrosome integrity and fertility of frozen ram spermatozoa treated with caffeine, pentoxifylline, cAMP, 2-deoxyadenosine and kallikrein. Reprod Fertil Dev. 1995;7(5):1081–7.
Milani C, Fontbonne A, Sellem E, Stelletta C, Gerard O, Romagnoli S. Effect of post-thaw dilution with caffeine, pentoxifylline, 2′-deoxyadenosine and prostatic fluid on motility of frozen-thawed dog semen. Theriogenology. 2010;74(1):153–64. https://doi.org/10.1016/j.theriogenology.2010.01.026.
Tesarik J, Mendoza C, Carreras A. Effects of phosphodiesterase inhibitors caffeine and pentoxifylline on spontaneous and stimulus-induced acrosome reactions in human sperm. Fertil Steril. 1992;58(6):1185–90.
Francis SH, Houslay MD, Conti M. Phosphodiesterase inhibitors: factors that influence potency, selectivity, and action. Handb Exp Pharmacol. 2011;204:47–84. https://doi.org/10.1007/978-3-642-17969-3_2.
Tournaye H, Janssens R, Verheyen G, Camus M, Devroey P, Van Steirteghem A. An indiscriminate use of pentoxifylline does not improve in-vitro fertilization in poor fertilizers. Hum Reprod. 1994;9(7):1289–92.
Yovich JL. Pentoxifylline: actions and applications in assisted reproduction. Hum Reprod. 1993;8(11):1786–91. https://doi.org/10.1093/oxfordjournals.humrep.a137935.
Wang R, Bell M, Hellstrom WJ, Sikka SC. Post-thaw sperm motility, cAMP concentration and membrane lipid peroxidation after stimulation with pentoxifylline and platelet-activating factor. Int J Androl. 1994;17(4):169–73.
Larsen L, Scheike T, Jensen TK, Bonde JP, Ernst E, Hjollund NH, et al. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. The Danish First Pregnancy Planner Study Team. Hum Reprod. 2000;15(7):1562–7. https://doi.org/10.1093/humrep/15.7.1562.
Morales P, Llanos M, Yovich JL, Cummins JM, Vigil P. Pentoxifylline increases sperm penetration into zona-free hamster oocytes without increasing the acrosome reaction. Andrologia. 1993;25(6):359–62. https://doi.org/10.1111/j.1439-0272.1993.tb02743.x.
Tasdemir M, Tasdemir I, Kodama H, Tanaka T. Pentoxifylline-enhanced acrosome reaction correlates with fertilization in vitro. Hum Reprod. 1993;8(12):2102–7.
Kay VJ, Coutts JR, Robertson L. Effects of pentoxifylline and progesterone on human sperm capacitation and acrosome reaction. Hum Reprod. 1994;9(12):2318–23. https://doi.org/10.1093/oxfordjournals.humrep.a138445.
Fisch JD, Behr B, Conti M. Enhancement of motility and acrosome reaction in human spermatozoa: differential activation by type-specific phosphodiesterase inhibitors. Hum Reprod. 1998;13(5):1248–54.
Hammadeh ME, Askari AS, Georg T, Rosenbaum P, Schmidt W. Effect of freeze-thawing procedure on chromatin stability, morphological alteration and membrane integrity of human spermatozoa in fertile and subfertile men. Int J Androl. 1999;22(3):155–62.
Kim SH, Yu DH, Kim YJ. Effects of cryopreservation on phosphatidylserine translocation, intracellular hydrogen peroxide, and DNA integrity in canine sperm. Theriogenology. 2010;73(3):282–92. https://doi.org/10.1016/j.theriogenology.2009.09.011.
Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl. 1992;13(3):232–41.
Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril. 1998;69(3):528–32. https://doi.org/10.1016/s0015-0282(97)00536-0.
Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14(4):1039–49. https://doi.org/10.1093/humrep/14.4.1039.
Gupta S, Sekhon LH, Agarwal A, editors. Sperm banking: when, why, and how? 2011.
Code availability
Text has been prepared using Microsoft Word application
Author information
Authors and Affiliations
Contributions
EI was involved in the sample collection, acquisition of data, patient’s enrollment, follow-up, and drafting of the article. SH performed statistical analysis. EB was involved in the interpretation of data, critical revision for important intellectual content, and drafting of the article. NG performed the design and interpretation of data. SO was involved in the conception, coordination and design of the study, acquisition of data, analysis, interpretation and critical revision of data, drafting of the article, and critical revision of the article for important intellectual content. All the authors performed the final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval
The approval of the Ankara University Research Ethical Committee was gained (Approval number: 02-60-18. Date: 22.01.2018).
Consent to participate
All subjects were informed with signed consent form prior to inclusion to the study, and the tenets of Declaration of Helsinki were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibis, E., Hayme, S., Baysal, E. et al. Efficacy and safety of papaverine as an in vitro motility enhancer on human spermatozoa. J Assist Reprod Genet 38, 1523–1537 (2021). https://doi.org/10.1007/s10815-021-02160-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02160-x