Abstract
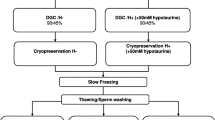
The primary objective of this study is to evaluate and to compare the effects of photobiomodulation (PBM) on sperm parameters both before and after cryopreservation. In this regard, 24 freshly ejaculated semen samples from normozoospermic men were included in this study. Each semen sample was randomly divided into three groups (1 ml aliquot for each group): the control group (group one) underwent conventional sperm cryopreservation (n = 24), group two underwent pre-freezing PBM exposure (810 nm, diode laser, and 0.6 J/cm2) (n = 24), and group three underwent post freezing and thawing PBM exposure (n = 24). Indicators of sperm quality, including total sperm motility (TSM), progressive sperm motility (PSM), DNA fragmentation, lipid peroxidation levels, apoptosis-like changes, and gene expression levels of protamine (PRM) 1, PRM2, and adducin 1 alpha (ADD1), were investigated in a blinded style. Due to the beneficial effect of pre-freezing PBM therapy, group 2 exhibited the highest TSM and PSM levels compared to groups 1 and 3. At the same time, DNA fragmentation and lipid peroxidation were significantly reduced in the group 2 compared to the group 1 (p = 0.024 p = 0.016, respectively). Evaluation of apoptotic/necrotic changes revealed that parameters including early apoptosis, dead, and necrotic cells decreased in the group 2 compared to the either groups 1 (p = 0. 008, p = 0. 032, p = 0. 02, respectively) or group 3 (p = 0.037, p = 0.108, p = 0.083). There were no significant differences in the expression levels of PRM1, PRM2, and ADD1 among the study groups. Based on our results, PBM therapy prior to cryopreservation, even in the normal semen samples, plays a significant protective role against cryo-damage by preserving the functional parameters of spermatozoa.





Similar content being viewed by others
Data Availability
Some more information about statistical analyses were provided, so if needed, we can send as supplementary of data.
Code Availability
All analyses were carried out using SPSS software (version 22 for Windows; SPSS Inc., Chicago, IL, USA).
References
Ravitsky V, Kimmins S. The forgotten men: rising rates of male infertility urgently require new approaches for its prevention, diagnosis and treatment. Biol Reprod. 2019;101(5):872–4.
Sinha S. Role of Cryopreservation in Assisted Reproductive Technology (ART). Apollo Medicine. 2009;6(3):212–21.
Di Santo M, Tarozzi N, Nadalini M, Borini A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol. 2011;2012.
Ezzati M, Shanehbandi D, Hamdi K, Rahbar S, Pashaiasl M. Influence of cryopreservation on structure and function of mammalian spermatozoa: an overview. Cell Tissue Bank. 2020;21(1):1–15.
Valcarce DG, Cartón-García F, Herráez MP, Robles V. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology. 2013;67(1):84–90.
Card CJ, Anderson EJ, Zamberlan S, Krieger KE, Kaproth M, Sartini BL. Cryopreserved bovine spermatozoal transcript profile as revealed by high-throughput ribonucleic acid sequencing. Biol Reprod. 2013;88(2):49.
Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, Guerin JF. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87(1):93–100.
Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015;21(2):209–27.
Kim GY. What should be done for men with sperm DNA fragmentation? Clin Exp Reprod Med. 2018;45(3):101–9.
Dai D-H, Qazi IH, Ran M-X, Liang K, Zhang Y, Zhang M, et al. Exploration of miRNA and mRNA profiles in fresh and frozen-thawed boar sperm by transcriptome and small RNA sequencing. Int J Mol Sci. 2019;20(4):802.
Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA, Network RM. The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update. 2013;19(6):604–24.
Amidi F, Pazhohan A, ShabaniNashtaei M, Khodarahmian M, Nekoonam S. The role of antioxidants in sperm freezing: a review. Cell Tissue Bank. 2016;17(4):745–56.
Bucak MN, Ateşşahin A, Varişli O, Yüce A, Tekin N, Akçay A. The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen Microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology. 2007;67(5):1060–7.
Fabozzi G, Starita MF, Rega E, Alteri A, Colicchia A, Piscitelli C, et al. Evaluation of the efficiency of two different freezing media and two different protocols to preserve human spermatozoa from cryoinjury. Int J Reprod Med. 2016;2016.
Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, Esmaeili V, et al. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 2018;37(3):327–39.
Hanna R, Agas D, Benedicenti S, Ferrando S, Laus F, Cuteri V, et al. A comparative study between the effectiveness of 980 nm photobiomodulation delivered by hand-piece with Gaussian vs. flat-top profiles on osteoblasts maturation. Front Endocrinol. 2019;10:92.
Zupin L, Pascolo L, Luppi S, Ottaviani G, Crovella S, Ricci G. Photobiomodulation therapy for male infertility. Lasers Med Sci. 2020;35(8):1671–80.
Gabel CP, Carroll J, Harrison K. Sperm motility is enhanced by low level laser and light emitting diode photobiomodulation with a dose-dependent response and differential effects in fresh and frozen samples. Laser Ther. 2018;27(2):131–6.
Preece D, Chow KW, Gomez-Godinez V, Gustafson K, Esener S, Ravida N, et al. Red light improves spermatozoa motility and does not induce oxidative DNA damage. Sci Rep. 2017;7:46480.
Safian F, Novin MG, Nazarian H, Mofarahe ZS, Abdollahifar M-A, Jajarmi V, et al. Photobiomodulation preconditioned human semen protects sperm cells against detrimental effects of cryopreservation. Cryobiology. 2020;98:239–44.
Lu J-C, Huang Y-F, Lü N-Q. WHO Laboratory Manual for the Examination and Processing of Human Semen: its applicability to andrology laboratories in China. Zhonghua Nan Ke Xue. 2010;16(10):867–71.
Dong L, Zhang X, Yang F, Li J, Yu X, Li Y. Effect of oral alpha-lipoic acid (ALA) on the treatment of male infertility: A protocol for systematic review and meta-analysis. Medicine. 2019;98(51).
Nabi A, Khalili M, Halvaei I, Roodbari F. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia. 2014;46(4):374–9.
Tavalaee M, Deemeh M, Arbabian M, Nasr-Esfahani M. Density gradient centrifugation before or after magnetic-activated cell sorting: which technique is more useful for clinical sperm selection? J Assist Reprod Genet. 2012;29(1):31–8.
Ostermeier GC, Goodrich RJ, Diamond MP, Dix DJ, Krawetz SA. Toward using stable spermatozoal RNAs for prognostic assessment of male factor fertility. Fertil Steril. 2005;83(6):1687–94.
Zeng C, He L, Peng W, Ding L, Tang K, Fang D, et al. Selection of optimal reference genes for quantitative RT-PCR studies of boar spermatozoa cryopreservation. Cryobiology. 2014;68(1):113–21.
Hatef B, Taromchi A, Nejatbakhsh R, Farrokhi A, Shokri S. Supplementation of freezing media with stromal cell-derived factor-1α preserves human sperm from cryodamage. Cryobiology. 2017;79:37–42.
Topraggaleh T, Shahverdi A, Rastegarnia A, Ebrahimi B, Shafiepour V, Sharbatoghli M, et al. Effect of cysteine and glutamine added to extender on post-thaw sperm functional parameters of buffalo bull. Andrologia. 2014;46(7):777–83.
Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, Esmaeili V, et al. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 2018;37(3):327–39.
Valcarce D, Cartón-García F, Herráez M, Robles V. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology. 2013;67(1):84–90.
García-Herrero S, Garrido N, Martínez-Conejero JA, Remohí J, Pellicer A, Meseguer M. Differential transcriptomic profile in spermatozoa achieving pregnancy or not via ICSI. Reprod Biomed Online. 2011;22(1):25–36.
Ortiz-Rodriguez JM, Ortega-Ferrusola C, Gil MC, Martín-Cano FE, Gaitskell-Phillips G, Rodríguez-Martínez H, et al. Transcriptome analysis reveals that fertilization with cryopreserved sperm downregulates genes relevant for early embryo development in the horse. PloS one. 2019;14(6):e0213420.
Rahiminia T, Hosseini A, Anvari M, Ghasemi-Esmailabad S, Talebi AR. Modern human sperm freezing: effect on DNA, chromatin and acrosome integrity. Taiwan J Obstet Gynecol. 2017;56(4):472–6.
Fernandes GH, de Carvalho Pde T, Serra AJ, Crespilho AM, Peron JP, Rossato C, et al. The effect of low-level laser irradiation on sperm motility, and integrity of the plasma membrane and acrosome in cryopreserved bovine sperm. PLoS One. 2015;10(3):e0121487.
Karu TI. Lasers in infertility treatment: irradiation of oocytes and spermatozoa. Photomed Laser Surg. 2012;30(5):239–41.
Lubart R, Friedmann H, Sinyakov M, Cohen N, Breitbart H. Changes in calcium transport in mammalian sperm mitochondria and plasma membranes caused by 780 nm irradiation. Lasers Surg Med. 1997;21(5):493–9.
Valcarce DG, Cartón-García F, Riesco MF, Herráez MP, Robles V. Analysis of DNA damage after human sperm cryopreservation in genes crucial for fertilization and early embryo development. Andrology. 2013;1(5):723–30.
Acknowledgements
The authors thank staffs of Andrology Laboratories of Assisted Reproduction Unit at Tleghani Hospital,Tehran, Iran, for their nice collaboration.
Funding
The present article was financially supported by Menʼs Health and Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences,Tehran, Iran (grant no: 21180).
Author information
Authors and Affiliations
Contributions
Dr Safian, Dr Ghaffari and Dr. Bayat wrote the proposal and manuscript. Dr Safian performed the methods. Dr Jajarmi, Dr Abdollahifar, Dr Nazarian, Dr Shams Mofarahe, and Dr Chien added some comments. Dr Kazemi and Dr Raee grammatically edited the paper. Dr Safian analyzed statistically the data. Dr Ghaffari did help in some methods.
Corresponding author
Ethics declarations
Ethical Approval and Consent to participate
It was mentioned in materials and methods.
Consent for Publication
It was not applicable.
Conflicts of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Safian, F., Bayat, M., Jajarmi, V. et al. Comparative Effect of Photobiomodulation on Human Semen Samples Pre- and Post-Cryopreservation. Reprod. Sci. 29, 1463–1470 (2022). https://doi.org/10.1007/s43032-021-00805-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00805-x