Abstract
Purpose
To evaluate a slow freezing method for whole ovary cryopreservation by evaluating effects of added cryoprotectant.
Methods
Sheep ovaries were isolated during surgery, flushed with either Ringer-Acetate or dimethylsulphoxide and cryopreserved by slow freezing. After rapid thawing, viability was assessed by ovarian in vitro perfusion, cell culture, histology and fluorescent live-dead assay.
Results
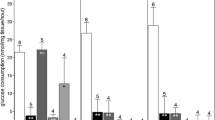
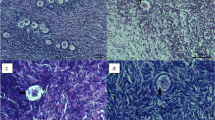
Production of cyclic AMP and progesterone was slightly higher in the dimethylsulphoxide group. Cultured ovarian cells from dimethylsulphoxide-preserved ovaries secreted larger amounts of progesterone than cells from Ringer-Acetate preserved. Light microscopy of ovarian biopsies obtained after perfusion, revealed well-preserved tissue in the dimethysulphoxide group but not in the Ringer-Acetate group. The density of small follicles and ovarian cell viability were higher in dimethysulphoxide ovaries compared to Ringer-Acetate ovaries.
Conclusions
Equilibrium with its protective effect can be achieved by slow freezing protocol, with an additional protective effect by the presence of dimethylsulphoxide.





Similar content being viewed by others
References
Marhhom E, Cohen I. Fertility preservation options for women with malignancies. Obstet Gynecol Surv. 2007;62:58–72.
Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113:2575–96.
Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422–34.
Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am J Epidemiol. 1999;150:245–54.
Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414.
Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010;93:762–8.
Bedaiwy MA, Hussein MR, Biscotti C, Falcone T. Cryopreservation of intact human ovary with its vascular pedicle. Hum Reprod. 2006;21:3258–69.
Candy CJ, Wood MJ, Whittingham DG. Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod. 2000;15:1300–4.
Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Hum Reprod. 2003;18:1165–72.
Chen CH, Chen SG, Wu GJ, Wang J, Yu CP, Liu JY. Autologous heterotopic transplantation of intact rabbit ovary after frozen banking at −196 degrees C. Fertil Steril. 2006;86:1059–66.
Gerritse R, Beerendonk CC, Tijink MS, Heetkamp A, Kremer JA, Braat DD, et al. Optimal perfusion of an intact ovary as a prerequisite for successful ovarian cryopreservation. Hum Reprod. 2008;23:329–35.
Imhof M, Hofstetter G, Bergmeister H, Rudas M, Kain R, Lipovac M, et al. Cryopreservation of a whole ovary as a strategy for restoring ovarian function. J Assist Reprod Genet. 2004;21:459–65.
Scott JR, Keye WR, Poulson AM, Reynolds WA. Microsurgical ovarian transplantation in the primate. Fertil Steril. 1981;36:512–5.
Bedaiwy MA, Jeremias E, Gurunluoglu R, Hussein MR, Siemianow M, Biscotti C, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003;79:594–602.
Arav A, Revel A, Nathan Y, Bor A, Gacitua H, Yavin S, et al. Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum Reprod. 2005;20:3554–9.
Imhof M, Bergmeister H, Lipovac M, Rudas M, Hofstetter G, Huber J. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril. 2006;85 Suppl 1:1208–15.
Bedaiwy MA, Falcone T. Harvesting and autotransplantation of vascularized ovarian grafts: approaches and techniques. Reprod Biomed Online. 2007;14:360–71.
Fahy GM, Wowk B, Wu J. Cryopreservation of complex systems: the missing link in the regenerative medicine supply chain. Rejuvenation Res. 2006;9:279–91.
Shaw JM, Jones GM. Terminology associated with vitrification and other cryopreservation procedures for oocytes and embryos. Hum Reprod Update. 2003;9:583–605.
Shaw JM, Oranratnachai A, Trounson AO. Fundamental cryobiology of mammalian oocytes and ovarian tissue. Theriogenology. 2000;53:59–72.
Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17:243–56.
McGann LE. Differing actions of penetrating and nonpenetrating cryoprotective agents. Cryobiology. 1978;15:382–90.
Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82:1390–4.
Wallin A, Ghahremani M, Dahm-Kahler P, Brannstrom M. Viability and function of the cryopreserved whole ovary: in vitro studies in the sheep. Hum Reprod. 2009;24:1684–94.
Wusteman MC, Pegg DE, Robinson MP, Wang LH, Fitch P. Vitrification media: toxicity, permeability, and dielectric properties. Cryobiology. 2002;44:24–37.
Dahm-Kahler P, Wranning C, Lundmark C, Enskog A, Molne J, Marcickiewicz J, et al. Transplantation of the uterus in sheep: methodology and early reperfusion events. J Obstet Gynaecol Res. 2008;34:784–93.
Bjersing L, Cajander S, Damber JE, Janson PO, Kallfelt B. The isolated perfused rabbit ovary-a model for studies of ovarian function. Ultrastructure after perfusion with different media. Cell Tissue Res. 1981;216:471–9.
Holmes PV, Hedin L, Janson PO. The role of cyclic adenosine 3′, 5′-monophosphate in the ovulatory process of the in vitro perfused rabbit ovary. Endocrinology. 1986;118:2195–202.
Runesson E, Ivarsson K, Janson PO, Brannstrom M. Gonadotropin- and cytokine-regulated expression of the chemokine interleukin 8 in the human preovulatory follicle of the menstrual cycle. J Clin Endocrinol Metab. 2000;85:4387–95.
Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Van Langendonckt A, Nottola SA, et al. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod. 2006;21:413–20.
Noyes N, Boldt J, Nagy ZP. Oocyte cryopreservation: is it time to remove its experimental label? J Assist Reprod Genet. 2010;27:69–74.
Varghese AC, Nagy ZP, Agarwal A. Current trends, biological foundations and future prospects of oocyte and embryo cryopreservation. Reprod Biomed Online. 2009;19:126–40.
Youssry M, Ozmen B, Zohni K, Diedrich K, Al-Hasani S. Current aspects of blastocyst cryopreservation. Reprod Biomed Online. 2008;16:311–20.
Munn CS, Kiser LC, Wetzner SM, Baer JE. Ovary volume in young and premenopausal adults: US determination. Work in progress. Radiology. 1986;159:731–2.
Newton H, Fisher J, Arnold JR, Pegg DE, Faddy MJ, Gosden RG. Permeation of human ovarian tissue with cryoprotective agents in preparation for cryopreservation. Hum Reprod. 1998;13:376–80.
Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, et al. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11:1268–72.
Demirci B, Lornage J, Salle B, Frappart L, Franck M, Guerin JF. Follicular viability and morphology of sheep ovaries after exposure to cryoprotectant and cryopreservation with different freezing protocols. Fertil Steril. 2001;75:754–62.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10.
Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–72.
Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006;85 Suppl 1:1150–6.
Brannstrom M, Flaherty S. Methodology and characterization of an in vitro perfusion model for the mouse ovary. J Reprod Fertil. 1995;105:177–83.
Abrahamsson G, Janson PO, Kullander S. An in vitro perfusion method for metabolic studies on human postmenopausal ovaries. Acta Obstet Gynecol Scand. 1990;69:527–32.
Toner M, Cravalho EG, Stachecki J, Fitzgerald T, Tompkins RG, Yarmush ML, et al. Nonequilibrium freezing of one-cell mouse embryos. Membrane integrity and developmental potential. Biophys J. 1993;64:1908–21.
Steponkus PL, Dowgert MF, Gordon-Kamm WJ. Destabilization of the plasma membrane of isolated plant protoplasts during a freeze-thaw cycle: the influence of cold acclimation. Cryobiology. 1983;20:448–65.
Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod. 1995;10:75–81.
Wadia PR, Mahale SD, Nandedkar TD. Effect of the human follicle-stimulating hormone-binding inhibitor and its N-terminal fragment on follicle-stimulating hormone-induced progesterone secretion by granulosa cells in vitro. J Biosci. 2007;32:1185–94.
Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, et al. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007;55:261–8.
Fauque P, Ben Amor A, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–7.
Onions VJ, Mitchell MR, Campbell BK, Webb R. Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod. 2008;23:606–18.
Acker JP, McGann LE. Innocuous intracellular ice improves survival of frozen cells. Cell Transplant. 2002;11:563–71.
Almici C, Ferremi P, Lanfranchi A, Ferrari E, Verardi R, Marini M, et al. Uncontrolled-rate freezing of peripheral blood progenitor cells allows successful engraftment by sparing primitive and committed hematopoietic progenitors. Haematologica. 2003;88:1390–5.
Acknowledgments
Financial support: Supported by grants from: Swedish Research Council, Stockholm, Sweden; Sahlgrenska Academy ALF, Göteborg, Sweden; Hjalmar Svensson’s Research Foundation, Göteborg, Sweden; and Assar Gabrielsson’s Research Foundation, Göteborg, Sweden.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule The study evaluates the impact of dimethylsulphoxide as a cryoprotectant for whole ovary cryopreservation.
Rights and permissions
About this article
Cite this article
Milenkovic, M., Wallin, A., Ghahremani, M. et al. Whole sheep ovary cryopreservation: evaluation of a slow freezing protocol with dimethylsulphoxide. J Assist Reprod Genet 28, 7–14 (2011). https://doi.org/10.1007/s10815-010-9477-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-010-9477-5