Abstract
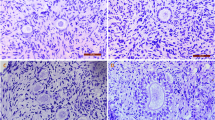
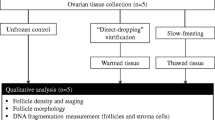
Cryopreservation of human ovarian tissue represents a key procedure for fertility preservation. The two most widely used cryopreservation methods for human ovarian cortex samples are slow freezing\thawing (SF\T) and vitrification\warming (V\W). The aim of the present study was to analyze the effects of SF\T and V\W using a metal chamber, on specific follicle and oocyte structures and on the stromal organization post-cryopreservation. We did histology analysis of SF\T and V\W ovarian fragments from nine healthy subjects. Overall results showed that cryopreserved tissues presented significant rates of damage in primordial and primary follicles. Altered nuclear structure of primordial follicles and cell detachment from primordial and primary follicles were the main injuries observed after V/W and SF/T. The stromal components were similarly well preserved after cryopreservation. We conclude that both cryopreservation methods may be used for fertility preservation purposes with similar outcomes in terms of follicular and stromal integrity. Detachment of follicle cells from basal membrane represents an important cryoinjury that deserves further investigation.


Similar content being viewed by others
Data Availability
All authors include information of no funding, non-financial interests, study-specific approval by the appropriate ethics committee for research involving humans (Conceição Hospital Group Ethics Committee approval #17,135) and informed consent as the research involved human participants (Informed consent was given and signed by all participants).
Code Availability
All data and materials as well as software application or custom code support our published claims and comply with field standards.
References
Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011; 8. https://doi.org/10.1186/1742-5573-8-1
Amorim CA, Curaba M, Van LA, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23:160–86. https://doi.org/10.1016/j.rbmo.2011.04.005.
Aquino D, Danielli L, Rigon P, Lothhammer N, Frantz N, Bos-Mikich A. Ovarian tissue vitrification: the use of a novel metal closed system for clinical grade cryopreservation. JBRA Assist Reprod. 2014;18:12–5. https://doi.org/10.5935/1518-0557.20140086.
Bos-Mikich A, Marques L, Rodrigues JL, Lothhammer N, Frantz N. The use of a metal containner for vitrification of mouse ovaries as a clinical grade model for human ovarian tissue cryopreservation after different times and temperatures of transport. J Assist Reprod Genet. 2012;29:1267–71. https://doi.org/10.1007/s10815-012-9867-y.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, Van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. https://doi.org/10.1016/S0140-6736(04)17222-X.
Fabbri R, Vicenti R, Macciocca M, Pasquinelli G, Paradisi R, Battaglia C, Martino NA, Venturoli S. Good preservation of stromal cells and no apoptosis in human ovarian tissue after vitrification. Biomed Res Int. 2014;2014:673537. https://doi.org/10.1155/2014/673537.
Fabbri R, Vicenti R, Macciocca M, Martino NA, Dell’Aquila ME, Pasquinelli G, Morselli-Labate AM, Seracchioli R, Paradisi R. Morphological, ultrastructural and functional imaging of frozen/thawed and vitrified/warmed human ovarian tissue retrieved from oncological patients. Hum Reprod. 2016;31:1838–49. https://doi.org/10.1093/humrep/dew134.
Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A, Winston RM. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11:1268–72. https://doi.org/10.1093/oxfordjournals.humrep.a019370.
Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol. 2008;139:193–8. https://doi.org/10.1016/j.ejogrb.2008.03.002.
Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, Bader M, Weiss JM. Human ovarian tissue vitrification versus conventional freezing: morphological and molecular biological evaluation. Reproduction. 2009;138:319–27. https://doi.org/10.1530/REP-09-0039.
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013;110:17474–9. https://doi.org/10.1073/2Fpnas.1312830110.
Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83. https://doi.org/10.1093/humrep/dep079.
Klocke S, Bundgen N, Koster F, Eichenlaub-Ritter U, Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet. 2015;291:419–26. https://doi.org/10.1007/s00404-014-3390-6.
Locatelli Y, Calais L, Duffard N, Lardic L, Monniaux D, Piver P, et al. In vitro survival of follicles in prepubertal ewe ovarian cortex cryopreserved by slow freezing or non-equilibrium vitrification. JARG. 2019;36:1823–35.
Mao M, Alavi MV, Labelle-Dumais C, Gould DB, in: J.H. Miner (Eds.) Current topics in membranes, Cambridge, 2015, pp. 61–116.
Margarone JE, Natiella JR, Vaughan CD. Artifacts in oral biopsy specimens. J Oral Maxillofac Surg. 1985;43:163–72. https://doi.org/10.1016/0278-2391(85)90154-5.
Marques LS, Rodrigues JL, Lothhammer N, Frantz N, Bös-Mikich A. Follicle and stroma integrity after holding ovarian tissue for up to 8 hours before vitrification in a metal closed system. JBRA Assist Reprod. 2013;17:169–72. https://doi.org/10.5935/1518-0557.20130056.
Martinez F. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril. 2017;108:407–15. https://doi.org/10.1016/j.fertnstert.2017.05.024.
Massignam ET, Ferreira M, Sanguinet E, Dupont A, Klamt F, Frantz N, Bös-Mikich A. Antioxidant defence capacity of ovarian tissue after vitrification in a metal closed system. JBRA Assist Reprod. 2018;22:199–204. https://doi.org/10.5935/2F1518-0557.20180044.
Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Ovary and ovulation: Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11:1487–91. https://doi.org/10.1093/oxfordjournals.humrep.a019423.
Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, Andersen AN, Andersen CY. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod Biomed Online. 2011;22:162–71. https://doi.org/10.1016/j.rbmo.2010.10.015.
Sanfilippo S, Canis M, Smitz J, Sion B, Darcha C, Janny L, Brugnon F. Vitrification of human ovarian tissue: a practical and relevant alternative to slow freezing. Reprod Biol Endocrinol. 2015;13:67. https://doi.org/10.1186/s12958-015-0065-5.
Santana LN, Van den Hurk R, Oskam IC, Brito AB, Brito DC, Domingues SFS, Santos RR. Vitrifcation of ovarian tissue from primates and domestic ruminants: an overview. Biopreserv Biobank. 2012;10:288–94. https://doi.org/10.1089/bio.2011.0048.
Schmidt KLT, Byskov AG, Nyboe Andersen A, Muller J, Yding AC. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–64. https://doi.org/10.1093/humrep/deg246.
Silber S, Fan Y, Goldsmith S. World wide update: results with cryopreserved ovarian tissue transplant. Fertil Steril. 2019;112:85. https://doi.org/10.1016/j.fertnstert.2019.07.342.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15. https://doi.org/10.1093/humrep/deu353.
Acknowledgements
We thank Mr Maikel Oliveira (Department of Morphological Sciences, ICBS, Federal University of Rio Grande do Sul) for helping with the histology procedures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The questionnaire and methodology for this study was approved by the Human Research Ethics committee of the Conceição Hospital Group (Ethics approval number:17135).
Consent to Participate
Written informed consent was obtained from the parents.
Consent for Publication
Patients signed informed consent regarding publishing their data and photographs.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramos, L., Galbinski, S., Nacul, A. et al. Detailed Morphological Analysis of Cryoinjury in Human Ovarian Tissue Following Vitrification or Slow Freezing. Reprod. Sci. 29, 2374–2381 (2022). https://doi.org/10.1007/s43032-021-00716-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00716-x