Abstract
A screening protocol was developed and applied to isolate and select cultivars of freshwater filamentous macroalgae for year-round monoculture cultivation and nutrient bioremediation of primary municipal wastewater. The screening protocol is a step-by-step guide to identify robust cultivars which possess key attributes of competitive dominance, high biomass productivity and bioremediation performance under local seasonal and extreme conditions. Forty-four mixed samples of freshwater filamentous macroalgae were collected during summer and winter from a range of local aquatic environments. Eleven isolated cultivars were grown in primary treated municipal wastewater and their biomass productivity and bioremediation performance under local ambient (summer and winter), extreme summer (max. summer) and winter (min. winter) conditions were assessed. Extreme conditions proved to be an important determining factor for cultivar selection as biomass productivity and bioremediation performance significantly declined under min. winter conditions. However, biomass productivity was not directly related to bioremediation performance, as cultivars with low growth rates maintained high nutrient removal rates under min. winter conditions. Top performing cultivars were Klebsormidium sp. (KLEB B) which reduced total ammoniacal-N concentrations by 99.9% to 0.01 mg L-1 (± 0.01 SE), Oedogonium sp. (OEDO D) which reduced nitrate-N concentrations by 90.2% to 0.08 mg L-1 (± 0.7 SE) and Rhizoclonium sp. which reduced phosphate concentrations by 98.7% to 0.02 mg L-1 (± 0.01 SE). Based on overall biomass productivity and bioremediation performance across seasonal and extreme conditions Klebsormidium sp. (KLEB B), Stigeoclonium sp. (STIG A) and Ulothrix sp. were identified as top performing cultivars suitable for the nutrient bioremediation of primary municipal wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater treatment plants (WWTPs) are point-sources for nutrient discharges entering into the aquatic environment (Carey and Migliaccio 2009; Arzate et al. 2019; van Puijenbroek et al. 2019). Consequently, there is an increasing need to upgrade existing WWTP infrastructure to achieve treatment standards, improve water quality of the receiving aquatic environment, and utilise primary municipal wastewater as a sustainable resource (De La Cueva Bueno et al. 2017; Salgot and Folch 2018; Chrispim et al. 2019). Filamentous algal bioremediation - the use of algae for the absorption and degradation of organic pollutants in aquatic environments (Baghour 2019) - can be a practical solution to upgrade municipal WWTP infrastructure (Cole et al. 2016; Lavrinovičs and Juhna 2017; Bhatt et al. 2021). Primary municipal wastewater is nutrient-rich (Simha and Ganesapillai 2017) providing a suitable medium to cultivate filamentous algae and thereby enable nutrient recovery through algal bioremediation (Renuka et al. 2013; Chrispim et al. 2019; Leong et al. 2021). Therefore, a high rate filamentous algal pond (HRFAP) system is low cost and simple to operate (Craggs et al. 2014; Garfí et al. 2017; Young et al. 2017), providing the added benefit of a consistent source of biomass for resource recovery and subsequent value-added products (Cole et al. 2016; Lawton et al. 2017; Leong et al. 2021). Algal bioremediation using HRFAPs could provide a circular economy approach to primary municipal wastewater treatment, converting a major waste stream into a commercially viable industry (Wreford et al. 2019; Kehrein et al. 2020; Catone et al. 2021; Škufca et al. 2021).
Monocultures are preferable for the effective treatment of primary municipal wastewater within a HRFAP to ensure bioremediation performance (Kebede-Westhead et al. 2006; Liu and Vyverman 2015; Liu et al. 2016; Lawton et al. 2021a) and consistent biomass composition for end-use product applications (Lawton et al. 2013, 2017; Neveux et al. 2016; Liu et al. 2020). Therefore, cultivar (a plant which has been cultivated by selective breeding) selection is a fundamental process in the initial production phase of any monoculture system (Borowitzka 2013). Cultivar selection should target native cultivars from within the local aquatic catchment or within the selected municipal WWTP (Kube et al. 2018; Bao et al. 2022) as the performance of a species or cultivar is dependent on genetic variability and the environment in which it grows naturally (Robinson et al. 2013). Consequently, cultivars which have adapted to nutrient-rich waters at municipal WWTPs may possess performance-enhancing traits which enable high biomass productivity and nutrient bioremediation performance (Kawecki and Ebert 2004; Cheregi et al. 2019; Pankratz et al. 2019). Targeting a native cultivar also avoids any potential biosecurity impacts incurred by the introduction of a foreign species. Introducing cultivars to new locations can have undesirable effects on local species and ecological processes (Ricciardi and Simberloff 2009; Champion 2018; Reid et al. 2019). Therefore, the WWTP was identified as a key site for sample collection.
Currently there is no standardised approach to screen local cultivars for a HRFAP monoculture system. To date, cultivars for filamentous algal bioremediation of municipal wastewater have been selected through laboratory-scale competition experiments under different seasonal conditions (Valero-Rodriguez et al. 2020), growth experiments within a variety of treated wastewaters (Ge et al. 2018), and assessment of biomass productivity and nutrient bioremediation performance in outdoor cultures in municipal wastewater (Lawton et al. 2021a). However, in all these studies cultivars were first pre-selected based on survival and scalability in standardised growth media under laboratory conditions. This cultivar pre-selection criterion may be appropriate for selecting cultivars for tertiary treated municipal wastewater, however, it is not effective when targeting primary municipal wastewater. This is because primary municipal wastewater contains nitrogen in the form of ammonia, which can be toxic to many algae when present at a high concentration (Wang et al. 2013; Ge et al. 2018). Ammonia concentrations in primary wastewater are also much higher than those found in standardised growth media and aquatic environments where algae typically grow (Ge and Champagne 2017; Karri et al. 2018). Consequently, only some cultivars can survive and be cultivated within primary municipal wastewater (Lu et al. 2018; Divya et al. 2023). Therefore, a standardised screening protocol must be specifically formulated to accurately identify local cultivars suitable for bioremediation of primary municipal wastewater.
An effective screening protocol must assess a range of attributes to determine the suitability of cultivars for nutrient bioremediation of primary municipal wastewater (Rani et al. 2021; Sameena et al. 2022). The screening protocol must identify cultivars which are competitively dominant to sustain monoculture production (Liu et al. 2020; Lawton et al. 2021b), thereby maintaining the quality of the biomass by remaining unialgal and uncontaminated (Sutherland and Ralph 2020). Primary municipal wastewater composition is nutrient-rich and highly variable depending on location and the type of wastewater influent entering the WWTP (Zhou et al. 2019). Thus, the screening protocol must ensure that selected cultivars can survive and maintain high biomass productivity when cultivated within primary wastewater from the targeted WWTP. Finally, bioremediation performance and biomass production can vary between seasons (Valero-Rodriguez et al. 2020). Therefore, a screening protocol needs to assess performance over a wide range of seasonal conditions (de Paula Silva et al. 2012; Lam and Lee 2014) to ensure water quality standards are met and production is maintained year-round (Stachowicz et al. 2008; Lawton et al. 2017).
The overall objective of this study was therefore to develop a screening protocol to select target cultivars of filamentous freshwater macroalgae for nutrient bioremediation of primary municipal wastewater. The specific aims were (i) to develop a screening protocol to identify and isolate cultivars from wild collected algal samples that are competitively dominant and fast-growing within the targeted medium; and (ii) compare nutrient bioremediation performance and biomass productivities of isolated cultivars grown in primary treated municipal wastewater under local seasonal conditions. It was hypothesised that cultivars collected from the WWTP, particularly from within primary wastewater clarifiers, would achieve high biomass productivity during acclimation and have high nutrient bioremediation performance during growth trials. Overall, this screening protocol was designed to select high performing cultivars which can then undergo further on-site pilot-scale trials to identify a single target cultivar suitable for year-round cultivation and nutrient bioremediation of primary municipal wastewater in a HRFAP monoculture.
Methods
Isolation and screening protocol
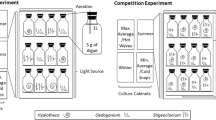
An isolation and screening protocol was developed to identify cultivars of naturally occurring filamentous freshwater macroalgae that are suitable for primary municipal wastewater bioremediation. This protocol included four key steps: sample collection, acclimation, growth trials, and bioremediation trials (Fig. 1). Each step is outlined in detail below. Briefly, mixed samples of filamentous algae were collected from local environments to target species which were acclimated to local climatic conditions. Samples were then maintained in culture under nutrient concentrations similar to primary wastewater for four weeks (acclimation). Based on acclimation performance, cultivars were selected for growth experiments which measured survival rate, biomass productivity and bioremediation performance in primary wastewater, and in response to seasonal variation in temperature and light.
Sample collection
Forty-four mixed samples of freshwater filamentous macroalgae were collected from the Te Puke WWTP, Aotearoa New Zealand, and aquatic environments in the vicinity of the plant including streams, agricultural drains, wetlands, and ephemeral water bodies (Appendix Table 4). Samples were collected during summer and winter to ensure the screening protocol included a range of species. Collected samples of < 2 g fresh weight (FW) were placed in 250 mL plastic containers in water from the collection site and stored in an insulated container and were transported to the University of Waikato Coastal Marine Field Station, Tauranga, Aotearoa New Zealand. Samples were maintained in their collection containers under constant laboratory conditions (18 °C) for 18 h. Any large debris were then manually removed, and the entire sample was poured through a 100 µm filter to remove excess suspended solids. The algal biomass was then divided into three replicate subsamples. Algal subsamples containing sufficient biomass were stocked at a rate of 1 g FW L-1 into separate 250 mL clear plastic containers with lids (LabServ) filled to 150 mL with filtered dechlorinated freshwater enriched with nutrients at a pH of 5.6 at 19.1 °C. Nutrient concentrations were based on primary wastewater concentrations of 5 mg NH4-N L-1 (NH4Cl), 1.3 mg PO4-P L-1 (NaH2PO4.2H2O) and trace metal concentrations (FeCl3.6H2O, C10H14N2Na2O8.2H2O, MnCl2.4H2O, ZnSO4.7H2O, CoCl2.6H2O, CuSO4.5H2O, Na2MoO4.2H2O) as per F/2 growth medium (Ryther and Guillard 1962; Guillard 1975). All growth media components were analytical grade and measured at a pH of 2.2 prior to dilution. Algal samples containing smaller quantities of biomass were separated into three large Petri dishes (Nest, 60 - 90 mm ø) until biomass had grown sufficiently to stock into 250 mL plastic containers at 1 g FW L-1. The acclimation step started immediately following this step.
Acclimation
The three replicate subsamples of each sample were maintained for four weeks under constant laboratory conditions (18 °C, 12:12 light:dark cycle at 60 - 100 µmol photons m-2 s-1 using cool fluorescent lights). Cultures were grown in the primary wastewater nutrient medium (as above). Cultures in 250 mL plastic containers were maintained in suspension by bubbling a constant gentle stream of filtered (Filtrec – FS134B8TI25 and Whatman Uniflo syringe filters, 0.22µm) air into each container. Cultures in Petri dishes were not aerated until biomass was upscaled into 250 mL plastic containers. Replicates were arranged on shelves in a randomized block design and were rotated daily within each block to minimise any edge effects and variation in light intensity. Biomass in each replicate container was harvested and culture medium was exchanged once every seven days over the four-week acclimation period. Biomass was harvested by mixing the contents of each container thoroughly to ensure that no algae had settled on the bottom of the container and then tipping the entire contents of each container (culture water and algae) into a fine mesh bag. Once excess water had drained from the bag, it was placed in a centrifugal spin dryer (Spindle NZ, SPL-265) and spun for approximately three minutes to remove any remaining water. The algae were then removed from the bag and weighed to determine the FW. Following harvesting, stocking density was reset to 1 g FW L-1 by restocking 0.15 g of the harvested biomass into each 150 mL replicate culture. Species within each sample were identified by morphological characteristics under the microscope (Olympus model CKX53) using freshwater algal identification guidebooks (Bellinger et al. 2010; John et al. 2011). Species composition in each replicate was estimated at the start and the end of the acclimation period by taking a small sample of the biomass from each replicate container and photographing ten sub-samples under a dissecting microscope (Olympus model CKX53) at 20 x magnification. Proportional species composition was estimated by placing a 100-point grid over each photo and summing the number of grid points directly overlying each species.
Cultivar isolation
Sixteen cultivars were selected for isolation and scale up based on their dominance in samples at the end of the acclimation period (Table 2). Approximately 20 - 30 individual filaments of each target cultivar were isolated from the mixed samples and placed into a sterile Petri dish filled with filtered dechlorinated freshwater, enriched with F/4 artificial growth medium at a pH of 5.7 at 17.4 °C (6.15 mg L-1 nitrate (NO3-), 0.56 mg L-1 phosphorus (PO4-), Rhyther and Guillard 1962; Guillard 1975). This process was repeated six times to establish six replicate petri dishes for each individual cultivar. Petri dishes were maintained under constant laboratory conditions (18 °C, 12:12 light:dark cycle at 50 µmol photons m-2 s-1 using cool fluorescent light). Nitrate (NO3-) was selected for upscaling isolated filaments, as previous attempts using ammonium (NH4-N) had caused algae to die-off. When the total biomass of each cultivar reached approximately 0.1 g FW, cultures were maintained with growth medium containing 5 mg NH4-N L-1 (NH4Cl), 1.3 mg PO4-P L-1 (NaH2PO4.2H2O) as described above and trace metals corresponding to rates found in F/2 medium (Ryther and Guillard 1962; Guillard 1975). Culture media were replaced weekly and cultivars were upscaled into larger petri dishes as biomass increased, then into 250 mL clear plastic containers and then into 2 L clear plastic containers. Seven of the 16 cultivars selected for isolation could not be scaled up due to slow growth and repeated issues with biomass cross-contamination of other cultivars and fungal infections.
Growth trials
Primary wastewater effluent
Growth trials were conducted to determine whether the nine isolated cultivars that were successfully scaled up could survive in diluted primary treated wastewater. Three additional cultivars that have been recently identified as targets for wastewater nutrient bioremediation were also included in the growth trials to provide a comparative measure of the performance of isolated cultivars. The three additional cultivars were an Oedogonium calcareum (OEDO A) cultivar from the University of Waikato Coastal Marine Field Station, Tauranga, New Zealand (Lawton et al. 2021a) and an Oedogonium sp. (OEDO B) and a Rhizoclonium sp. cultivar from the National Institute of Water and Atmospheric Research (NIWA), Hamilton, New Zealand (Hariz et al. 2022). Five replicate cultures of each cultivar (n = 60 cultures) were maintained in a temperature-controlled plant growth cabinet (Panasonic MLR-352, 18 °C, 12:12 light:dark cycle at 100 - 200 µmol photons m-2 s-1).
Experiments were conducted using free-floating (suspension) cultures of each cultivar in 250 mL plastic containers (LabServ) filled to 150 mL at a stocking density of 1g FW L-1. Cultures were maintained in suspension by bubbling a constant gentle stream of filtered (Resun LP-40 air pump and Whatman Uniflo syringe filters, 0.22 µm) air into each container. Cultures were grown in primary treated wastewater collected from the Te Puke WWTP during daily peak inflow. Wastewater was settled for 4 h in 20 L plastic buckets on collection. Physico-chemical parameters of the primary wastewater were measured at a pH of 7.5, temperature of 22.4°C, biological oxygen demand (BOD5) of 310 mg L-1 and chemical oxygen demand (COD) of 750 mg L-1. These analyses were conducted following standard methodology by Tauranga City Council Laboratory, Tauranga, New Zealand. Supernatant primary wastewater was then removed from the top of the bucket and diluted at a ratio of 1:3 with filtered dechlorinated freshwater (i.e., 25% wastewater). Wastewater was diluted to simulate wastewater concentrations within a HRAP where there would be a constant low flow of wastewater entering the HRAP, mixing with pre-existing partially treated wastewater. At each water collection, three additional 500 mL water samples were collected post-dilution and physico-chemical parameters were measured as total suspended solids (TSS) at 64 – 116 mg L-1, total Kjeldahl nitrogen (TKN) at 14.6 – 15.5 mg L-1, total ammoniacal-N (TAN) at 9.7 – 11.4 mg L-1, nitrate-N + nitrite-N at 0.50 – 0.75 mg L-1, dissolved reactive phosphorus at 0.96 – 1.31 mg L-1 and total phosphorus at 2.5 -3.1 mg L-1. These analyses were conducted following standard methodology by Hill Laboratories in Hamilton, New Zealand. Cultures were arranged within the plant growth cabinet using a randomized block design where one replicate of each cultivar was placed on each shelf of the cabinet and replicates within a shelf were rotated daily to minimise any edge effects.
The experiment was run for 14 days and biomass in each replicate container was harvested on day 7 and day 14 as described above. Total suspended solids and bacterial loads declined within the first 24-h period, as no bacterial flocs were present. Once harvested, the culture medium in each container was replaced with new diluted primary wastewater from the Te Puke WWTP and stocking density was reset to 1 g FW L-1 by restocking 0.15 g of the harvested biomass back into each 150 mL replicate culture. Excess biomass not restocked back into containers at day 7 from each replicate culture and all biomass on day 14 from each replicate culture was dried in an oven at 60 °C for 48 h and reweighed to determine the fresh weight to dry weight (FW:DW) ratio for each replicate. FW:DW ratios were used to convert the initial biomass and the harvested biomass for each replicate, which were both measured in FW, into DW. Biomass productivity (g DW m-2 day-1) was calculated for each replicate for each harvest using the equation P = (Bf – Bi)/ A / T, where Bf and Bi are the final and initial algal biomasses (g DW), A is the area (m2) of culture container and T is the culture period (7 days).
Seasonal variation
To identify top performing cultivars for year-round cultivation, cultivars which survived cultivation in primary wastewater effluent (n = 8) and the three additional cultivars were then maintained under temperatures and light intensities representative of local summer and winter conditions. Four experiments were conducted to represent ambient summer, ambient winter, maximum summer (max. summer) and minimum winter (min. winter) conditions. Methods were identical for each experiment, with the exception of temperature and light settings. Conditions were based on the National Climate Database weather recording station located in Te Puke (-37.82455, 176.32048, data available from www.cliflo.niwa.co.nz). Temperature profiles were based on the average high and average low temperature from the previous January for summer ambient conditions, and from the previous July for winter ambient conditions (Table 1, Figure 2a). Temperature profiles for max. summer conditions were based on the day of the previous year with the highest recorded summer temperature, using the average highest temperature recorded over a 6-h period of that day and the average temperature of the remaining 18-h period to provide a maximum and minimum temperature respectively (Table 1, Figure 2a). Temperature profiles for min. winter conditions were based on the day of the previous year with the lowest recorded winter temperature, using the average lowest temperature recorded over a 6-h period of that day and the average temperature of the remaining 18-h period to provide a minimum and maximum temperature respectively (Table 1, Figure 2a). The same light profiles were used for both the ambient and maximum/minimum experiments. These were based on the average daily light data recorded from the previous January for summer conditions and from the previous July for winter conditions (Table 1, Figure 2b).
Five replicate cultures of each cultivar (n = 55 cultures in total) were maintained in a temperature-controlled plant growth cabinet (Panasonic MLR-352) for each experiment. Cultures were maintained for 21 days for each of the summer and winter experiments with a harvest every seven days. Cultures were then maintained in the cabinets for a further four days under max. summer conditions immediately following the summer experiment and under min. winter conditions immediately following the winter experiment. Experiments were conducted using the same methodology described above in the primary wastewater effluent growth trial with the exception of temperature and light conditions.
A pulse amplitude-modulated (PAM) fluorometer (Junior-PAM, Heinz Walz GmbH, Germany) was used to measure the maximal quantum yield (Fv/Fm) in each replicate at each harvest. Fv/Fm was measured as an estimate of the maximal photochemical PSII efficiency and to indicate the presence of stress in the algal cultures (Kromkamp et al. 2008; Figueroa et al. 2013). Measurements were taken immediately before each harvest (i.e. once every seven days) at approximately the same time for each experiment on biomass samples that had been dark-adapted for 15 min (Stirbet 2011).
The bioremediation capabilities of cultivars in the seasonal growth trials were quantified by measuring concentrations of total ammoniacal-N (TAN), nitrate-N, and phosphate in the initial and final wastewater of the final seven-day cultivation cycle for the summer and winter experiments, and for the max. summer and min. winter experiments. A 150 mL sample of diluted filtered primary wastewater was collected at the initial restock of each cultivation cycle on day 14 and day 21, and from each replicate at harvest on day 21 and day 25. All wastewater samples were stored in individual 250 mL clear plastic containers with lids (LabServ). Total ammoniacal-N (sum of both molecular ammonia NH3 and ionic ammonium NH4+), nitrate-N and phosphate concentrations in each sample were measured using a HACH 900 (HACH, USA) spectrophotometer using the USEPA Nessler method (HACH method 8038), the nitrate-N cadmium reduction method (HACH method 8039), and the ascorbic acid method (HACH method 8048), respectively.
Statistical analysis
Biomass productivity and optimal quantum yield (Fv/Fm) measurements for summer and winter experiments were analysed using two factor repeated-measures analyses of variance (ANOVA) with cultivars and harvests as fixed factors. Biomass productivity and optimal quantum yield (Fv/Fm) measurements for max. summer and min. winter experiments were analysed using a one-way ANOVA with cultivars as a fixed factor. Nutrient concentrations for summer/max. summer experiments and winter/min. winter experiments were analysed using a one-way ANOVA with cultivars as a fixed factor. Data for each experiment were analysed separately. Normality was assessed using the Shapiro-Wilks normality test. All analyses were conducted in SPSS Statistics (version 29). All data are reported as means ± S.E.
Results
Species collection, acclimation, and cultivar isolation
Of the 44 samples collected, 18 samples completed the acclimation step, providing a total of 66 species from 12 genera (Table 2). A greater number of samples were collected in summer (30 samples) compared to winter (14 samples). However, the range of species present across all samples collected within a season did not vary between seasons and an equal number of species from both seasons were successfully upscaled following acclimation. Summer species were primarily collected from the WWTP, while winter species were generally collected from wetlands. Most species that were found in river habitats had low survival rates. The most common genus identified within acclimated samples from summer and winter was Oedogonium with 12 isolates identified in samples collected from a range of habitats including drainage channels, wetlands, and within the WWTP. Stigeoclonium was also commonly found, with 11 isolates collected from a range of habitats. During acclimation, Klebsormidium, Oedogonium, Stigeoclonium, and Ulothrix were competitively dominant within the mixed sample cultures (Table 2). Following acclimation, 19 species were isolated and of these, nine species from six genera were further upscaled. Cultivars of Klebsormidium sp. and Cladophora sp. collected in the summer had the highest growth rate during the isolation and upscale process.
Biomass productivity and photosynthetic efficiency
Biomass productivity varied significantly among cultivars and harvests under both summer and winter conditions, however, the cultivar with the highest productivity varied between harvests (Two-way ANOVA: summer: cultivar × harvest, F20,88 = 30.1, p = <0.001, winter: cultivar × harvest, F20,88 = 5.2, p = <0.001). Biomass productivity also varied significantly among cultivars under both max. summer (One-way ANOVA: F9,40 = 64.1, p = <0.001) and min. winter conditions (One-way ANOVA: F10,44 = 7.3, p = <0.001). Biomass productivity was 233 % higher on average across all cultivars under summer conditions compared to winter conditions, followed by max. summer, winter and min. winter conditions (Fig. 3a). Oedogonium sp. (OEDO A) had the highest productivity under summer conditions at 6.85 g m-2 DW day-1 (± 0.36) and Oedogonium sp. (OEDO C) had the highest productivity under max. summer conditions at 7.29 g m-2 DW day-1 (± 0.28). Klebsormidium sp. (KLEB B) had the highest productivity under winter conditions at 2.01 g m-2 DW day-1 (± 0.36) and Stigeoclonium sp. (STIG A) had the highest productivities under min. winter conditions at 1.65 g m-2 DW day-1 (± 0.32). Average biomass productivity across all cultivars declined under max. summer conditions by 12% compared to summer conditions. All cultivars except Klebsormidium sp. (KLEB A), Oedogonium sp. (OEDO A, OEDO C, OEDO D) and Ulothrix sp. declined in biomass productivity under max. summer conditions compared to summer conditions. Similarly, average biomass productivity across all cultivars declined under min. winter conditions by 92% compared to winter conditions. All cultivars except for Klebsormidium sp. (KLEB B), Oedogonium sp. (OEDO B), Stigeoclonium sp. (STIG A, STIG B) and Ulothrix sp. (Table 3) had biomass die-off under min. winter conditions.
Mean (±S.E.) biomass productivity (a) and optimal quantum yield (Fm/Fv) of chlorophyll a fluorescence (b) of 11 cultivars of freshwater filamentous macroalgae during the four seasonal light and temperature treatments. Data are averages of all three harvests for summer and winter experiments, and one harvest for max summer and min winter experiments, N = 5
Optimal quantum yields varied significantly among harvests; however, this variation was not consistent among cultivars under both summer and winter conditions (Two-way ANOVA: summer: cultivar × harvest, F30,132 = 3.5, p = <0.001, winter: cultivar × harvest, F30,132 = 5.8, p = <0.001). Optimal quantum yields also varied significantly among cultivars under max. summer and min. winter conditions (One-way ANOVA: max. summer: cultivar, F9,40 = 7.5, p = <0.001, min. winter: cultivar, F10,44 = 59.7, p = <0.001) (Fig. 3b). Across all cultivars, optimal quantum yields were the highest on average under summer conditions (0.7) and lowest on average under min. winter conditions (0.6).
Bioremediation performance
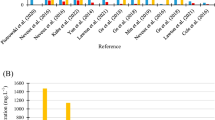
Total ammoniacal-N concentrations within the diluted primary wastewater (ratio of 1:3 primary wastewater:freshwater) across all seasons ranged between 10.0 – 11.1 mg L-1. Total ammoniacal-Nconcentrations in the primary wastewater post-harvest varied significantly among cultivars under all seasonal conditions (One-way ANOVA: summer: F10,44 = 12.2, p = <0.001, max. Summer: F10,44 = 6.4 p = <0.001, winter: F10,44 = 12.2, p = <0.001, min. Winter: F10,44 = 85.8, p = <0.001, Fig. 4a, Table 3). Across all cultivars, reductions in TAN were higher on average under summer conditions (92.4%) and max. summer conditions (99.1%), compared to colder conditions where there was a decline in TAN of 71.2% on average across cultivars under winter conditions and 45.1% under min. winter conditions. Cladophora sp. achieved the highest bioremediation performance under summer and max. summer conditions, reducing TAN concentrations by 94.7% to 0.56 mg L-1 (± 0.05) and 99.8% to 0.02 mg L-1 (± 0.01), respectively. Klebsormidium sp. (KLEB B, KLEB A) achieved the highest bioremediation performance under winter and min. winter conditions, reducing TAN concentrations by 99.9% to 0.01 mg L-1 (± 0.01) and 93.1% to 0.72 mg L-1 (± 0.06), respectively. Across all seasons, Klebsormidium sp. (KLEB B) had the highest bioremediation performance on average, reducing TAN by 94.8% to 0.6 mg L-1 (± 0.35).
Mean (±S.E.) total ammoniacal-N (TAN % Removal) (a), total ammoniacal-N + nitrate-N ( TAN + NO3-N % Removal) (b), and phosphate (PO43ˉ % Removal) (c), nitrate-N (NO3-N % Removal) (d) percentage of removal in the culture water of 11 cultivars of freshwater filamentous macroalgae when grown under four seasonal light and temperature treatments. N = 5
Nitrate-N concentrations within the diluted primary wastewater (ratio of 1:3 primary wastewater:freshwater), across all seasons ranged between 0.8 – 1.1 mg L-1. Nitrate-N concentrations in the primary wastewater post-harvest varied significantly among cultivars under all seasonal conditions (One-way ANOVA: summer: F10,44 = 6.2, p = <0.001, max. summer: F10,44 = 3.6, p = 0.001, winter: F10,44 = 6.2, p = <0.001, min. winter: F10,44 = 4.1, p = 0.001, Figure 4d, Table 3). Across all cultivars, reductions in nitrate-N were higher on average under summer conditions (64.8%) and max. summer conditions (52.8%) compared to colder conditions where there was an increase in nitrate-N concentrations of 35.8% on average across cultivars under winter conditions and 177.5% under min. winter conditions. Oedogonium sp. (OEDO A) achieved the highest bioremediation performance under summer conditions, reducing nitrate-N concentrations by 89.8% to 0.11 mg L-1 (± 0.05) and Oedogonium sp. (OEDO D) reduced nitrate-N concentrations under max. summer conditions by 90.2% to 0.08 mg L-1 (± 0.07). Klebsormidium sp. (KLEB B) achieved the highest reduction in nitrate-N concentrations under winter conditions by 63.9% to 0.34 mg L-1 (± 0.10). All cultivars had an increase in nitrate-N under min. winter conditions except Cladophora sp. which reduced nitrate-N concentrations by 27.3% to 0.54 mg L-1 (± 0.21). However, all cultivars under min. winter conditions showed a reduction in combined concentrations of TAN and nitrate-N except for Rhizoclonium sp. and Oedogonium sp. (OEDO A, OEDO B) (Fig. 4b). Across all seasons, Klebsormidium sp. (KLEB B) had the highest bioremediation performance on average, reducing nitrate-N by 34% to 0.59 mg L-1 (± 0.27).
Phosphate concentrations within the diluted primary wastewater (ratio of 1:3 primary wastewater:freshwater), across all seasons ranged between 1.17 – 1.41 mg L-1. Phosphate concentrations in the primary wastewater post-harvest varied significantly among cultivars under all seasonal conditions (One-way ANOVA: summer: F10,44 = 3.5, p = <0.001, max. summer: F10,44 = 73.3, p = <0.001, winter: F10,44 = 3.5, p = <0.001, min. winter: F10,44 = 40.6, p = <0.001, Figure 4c, Table 3). Across all cultivars, reductions in phosphate were highest on average under summer conditions (95.9%), comparable under max. summer (87.0%) and winter conditions (86.8%) and lowest under min. winter conditions (60.2%). Rhizoclonium sp. (RHIZ) achieved the highest reduction in phosphate concentrations under summer conditions by 98.7% to 0.02 mg L-1 (± 0.01). Cladophora sp. achieved the highest reduction in phosphate concentrations under max. summer conditions by 98.2% to 0.02 mg L-1 (± 0.01). Oedogonium sp. (OEDO C) achieved the highest reduction in phosphate concentrations under winter conditions by 93.6% to 0.06 mg L-1 (± 0.03) and Oedogonium sp. (OEDO B) achieved the highest reduction in phosphate concentrations under min. winter conditions by 94.9% to 0.09 mg L-1 (± 0.03). Across all seasons, Oedogonium sp. (OEDO B) had the highest bioremediation performance on average, reducing phosphate by 94.3% to 0.07 mg L-1 (± 0.03).
Discussion
A screening protocol was developed as a step-by-step guide and successfully applied to select freshwater filamentous macroalgal cultivars for nutrient bioremediation of primary municipal wastewater. As cultivar performance is dependent on genetic variability and the environment in which it grows naturally (Robinson et al. 2013), the WWTP was identified as a key habitat for sample collection. However, contrary to expectations, the cultivars which exhibited high biomass productivities during acclimation were collected from a range of habitats (e.g., agricultural drains, wetlands) as well as from the WWTP. Moreover, only four of the 11 cultivars collected from the WWTP progressed through to cultivar isolation and growth trials (Cladophora sp., Klebsormidium sp. (KLEB A), Stigeoclonium sp. (STIG A) and Ulothrix sp.) and only two of those cultivars demonstrated high productivity and nutrient bioremediation performance across all seasons (Stigeoclonium sp. (STIG A) and Ulothrix sp.). This poorer than expected performance of cultivars collected from the WWTP could be due to the change in cultivation conditions between the WWTP and the acclimation step. The samples from the WWTP were collected from areas of low-flow wastewater where they were growing attached to a substrate. In contrast, during the acclimation samples were cultivated on artificial growth media in free floating (suspension) cultures under laboratory conditions. It is possible therefore that substrate and physiochemical conditions could have reduced the number of cultivars which were able to grow during the acclimation period (Pikosz et al. 2017). However, the acclimation may have provided the opportunity for cultivars from various aquatic environments (e.g., wetlands) to dominate as increased nutrient concentrations generally stimulate growth of opportunistic algae (Krause-Jensen et al. 2007). The results of this study demonstrate that nutrient-rich aquatic environments such as WWTP’s should not be assumed to produce cultivars which are suitable for nutrient removal of primary wastewater. Instead, various local aquatic environments beyond WWTP’s, such as agricultural drains and wetlands, should also be sampled and surveyed to identify cultivars which exhibit superior biomass productivity and nutrient bioremediation performance in primary wastewater.
Optimum light and temperature conditions for algal growth are species-specific (Yun et al. 2014; Vadeboncoeur et al. 2021). Nutrient bioremediation performance of individual cultivars may also vary from season to season (Nguyen et al. 2022) and previous research has recommended that bi-cultures (Li et al. 2021) or monocultures with seasonal rotation of dominant cultivars is required to maximise stable biomass production for year-round cultivation (Valero-Rodriguez et al. 2020). Therefore, sample collection was undertaken during summer and winter to ensure a wide range of cultivars were collected that could grow under varying light and temperature conditions and increase the potential of identifying dominant cultivars for year-round nutrient bioremediation of primary wastewater. However, the same key genera were present in samples during summer and winter collections, with an equal number of cultivars from both seasons further selected for cultivar isolation and growth trials. Cultivars from the growth trials which demonstrated high nutrient bioremediation performance and biomass productivity were also collected during both seasons. However, in some instances, cultivars did not perform better under the seasonal conditions that corresponded to the season in which they were collected. For example, during the growth trial under winter conditions, Klebsormidium sp. and Ulothrix sp. collected in summer outperformed cultivars collected during winter in both biomass productivity and bioremediation performance. Biomass productivity throughout growth trials was highest under summer conditions across all cultivars regardless of which season samples were collected. In combination, these findings suggest that single season sampling may be sufficient to capture a diverse range of cultivars with the added benefit of saving on time and resources. However, if possible, and particularly in cases where diversity is low, it is important to sample during summer and winter to increase the number of samples collected and subsequently increase the probability of identifying a superior cultivar.
Monocultures require a cultivar to be competitively dominant, with high tolerance and adaptability to varying environmental conditions (Borowitzka 2013; Liu et al. 2020). Therefore, the acclimation step was designed to identify cultivars that are competitively dominant when grown in a nutrient medium with high concentrations of ammonia-N comparable to a constant low flow of primary municipal wastewater. Notably, this medium differs significantly from the environment in which most cultivars would naturally occur. Patterns of cultivar dominance and response to the growth medium varied during the acclimation step. Some cultivars remained dominant from the time of collection through to the end of acclimation. Other cultivars which were not detected in the initial samples became abundant during acclimation and, in some cases, dominant upon completion of this phase. This occurred in a total of 10 samples collected across various sampling sites and was most common in cultivars from the genera Oedogonium, Stigeoclonium, and Ulothrix. This response is known to occur in natural environments where species lay dormant until favourable conditions arise (Brawley and Johnson 1992). These findings indicate that cultivars which demonstrate competitive dominance in their natural habitat or upon sample collection may not remain dominant once acclimated within a nutrient medium with high concentrations of ammonia-N. Therefore, an acclimation period using a nutrient medium with high concentrations of ammonia-N is critical to identify cultivars that will be competitively dominant in nutrient-rich primary wastewater.
Nutrient bioremediation of TAN is a key indicator of algal bioremediation performance in primary wastewater where nitrogen exists mainly in the form of ammonium (NH4+) along with low levels of nitrate (NO3-) and nitrite (NO2-) (Tchobanoglous et al. 2003; Ge and Champagne 2017). Algae can utilize different forms of nitrogen; however, the preferred form of nitrogen differs between species (Silkina et al. 2017; Salbitani and Carfagna 2021). Consequently, cultivar uptake rates of TAN and nitrate-N may vary, as was found during growth trials with significant differences in uptake rates between cultivars in each season. Cultivar biomass productivity would also affect nitrogen assimilation across seasons. Estimations of nitrogen assimilation by Klebsormidium and Oedogonium cultivars were determined based on the average dry weight nitrogen content of 6.3% for Klebsormidium and 6.7% for Oedogonium (Lawton et al. 2021a). Estimations show that average nitrogen assimilation rates of Klebsormidium (summer: 78%, winter: 32%) and Oedogonium (summer: 94% and winter: 27%) declined by 59% and 71%, retrospectively between summer and winter. Consequently, the lower TAN and nitrate-N removal rates recorded for most cultivars during the winter growth trials compared to summer growth trials, were most likely due to lower algal assimilation as a result of low growth and survivorship (Fig. 5). An unexpected result during winter growth trials was the higher concentration of nitrate-N measured in the diluted primary wastewater at the time of harvest compared to the beginning of the growth trial. Since assimilation of nutrients by algae declines as rates of photosynthesis reduce under colder and lower light conditions in winter, nitrate-N assimilation was likely slower than the rate of nitrate-N generation by bacterial nitrification, contributing to the higher nitrate-N concentrations at the time of harvest. These results were further exacerbated under min. winter conditions, as a further reduction in photosynthesis further reduced the assimilation of nutrients. Nitrate-N concentrations may have also increased under winter and min. winter conditions due to the aeration supplied to the culture vessels, further promoting nitrification (Lehtovirta-Morley 2018; Cruz et al. 2019). However, across seasons biomass productivity did not directly relate to bioremediation performance as cultivars with low growth rates maintained high nutrient removal rates under min. winter conditions. These findings highlight the need to measure biomass productivity and nutrient bioremediation performance under a range of seasonal conditions to manage expectations around minimum bioremediation performance and allow design of management options for extreme weather conditions. Higher nutrient removal under summer conditions was most likely due to algal assimilation and ammonia volatilisation (Fig. 5), which occurs when algal photosynthetic activity elevates daytime pH above 9.0 (Park and Craggs 2011; Craggs et al. 2014). During summer an additional consideration is the reduced activity of nitrifying bacteria. These are highly sensitive to pH and are inhibited at pH > 9.0 (Sutherland et al. 2015). Consequently, nitrification can become inhibited under summer conditions due to high daytime culture pH, leading to relatively higher concentrations of ammonia-N than nitrate-N in summer compared to winter. This highlights the need to measure both TAN and nitrate-N/nitrite-N during seasonal growth trials to ensure that correct estimates of the total amount of dissolved inorganic nitrogen bioremediation are obtained.
Previous studies assessing bioremediation performance of common macroalgal genera have identified Oedogonium as a target genus for nutrient bioremediation of municipal wastewater (Lawton et al. 2013, 2021a). Two cultivars of Oedogonium (OEDO A, OEDO C) achieved the highest biomass productivity under summer and max. summer conditions. Two cultivars of Oedogonium (OEDO A, ODEO D) also achieved the highest bioremediation of nitrate-N under summer and max. summer conditions. However, nutrient bioremediation and growth of all Oedogonium sp. declined under winter and min. winter conditions (1.92 to -0.60 g m-2 DW day-1) compared to summer and max. summer conditions (7.29 to 2.10 g m-2 DW day-1). These findings correspond to previous studies which have identified Oedogonium sp. as a dominant summer cultivar with high biomass productivity (Hariz et al. 2022; 2023) and high removal of nitrate-N in wastewater effluent under summer conditions (Lawton et al. 2021a), and variable growth and biomass die off during colder conditions (< 10°C) (Lawton et al. 2014; Cole et al. 2018). Klebsormidium sp. (KLEB B) and Stigeoclonium sp. (STIG A) achieved the highest biomass productivity under winter and min. winter conditions, respectively. Klebsormidium sp. (KLEB B, KLEB A) also achieved the highest bioremediation of TAN under both winter and min. winter conditions in addition to the highest nitrate-N removal under winter conditions. Klebsormidium sp. and Stigeoclonium sp. are known to be highly tolerant of colder climates and often outperform other cultivars during winter and min. winter conditions (Borchhardt and Gründling-Pfaff 2020). Cladophora sp. achieved the highest removal of TAN under summer and max. summer conditions and was the only cultivar that reduced nitrate-N concentrations under min. winter conditions. Previous research has recognised Cladophora sp. for year-round growth and ability to uptake different forms of nitrogen from wastewater (Ross et al. 2018). Overall, Klebsormidium sp. (KLEB B), Stigeoclonium sp. (STIG A) and Ulothrix sp. were identified as top performing cultivars suitable for the year-round nutrient bioremediation of primary municipal wastewater based on their ability to maintain high growth rates and high nutrient removal rates across all seasons.
This is the first screening protocol to be developed to select target cultivars of filamentous freshwater macroalgae for bioremediation of primary municipal wastewater. The screening protocol ensures applicability and consistency from the point of sample collection to completion of growth trials by providing a step-by-step guide to identify target cultivars. The simple methodology outlined within the protocol allows for ease of replication. Each phase of the protocol uses cost effective and easily accessible experimental equipment. Sampling locations are the decision of the individual conducting the protocol rather than a feature of the protocol itself and thus the protocol is therefore inclusive of all local habitats. The protocol ensures that the target cultivar is robust and possesses key attributes e.g., competitive dominance, high biomass productivity and bioremediation performance under local, seasonal, and extreme conditions. Overall, this screening protocol has been proven successful through application to identify target cultivars which can maintain a monoculture for year-round nutrient bioremediation of primary municipal wastewater.
Conclusion
The screening protocol outlined in this study successfully identified filamentous freshwater macroalgal cultivars for nutrient bioremediation of primary municipal wastewater. The screening protocol identified the need to sample from a range of habitats, to undertake growth trials under extreme seasonal conditions and measure TAN and nitrate-N concentrations when analysing bioremediation performance. In general, biomass productivity and bioremediation performance were highest across all cultivars under summer and max. summer conditions. However, biomass productivity was not directly related to bioremediation performance. Based on overall growth and nutrient bioremediation performance under all seasonal and extreme conditions, the screening protocol successfully identified Klebsormidium sp. (KLEB B), Stigeoclonium sp. (STIG A) and Ulothrix sp. as potential candidates for year-round monoculture cultivation for nutrient bioremediation of primary municipal wastewater. However, HRFAP environments are highly-variable and cultivar performance under laboratory conditions is not always indicative of cultivar performance within outdoor systems. Therefore, the next step to confirm the suitability of these cultivars is to conduct competition experiments in outdoor pond trials in primary municipal wastewater to ensure that cultivars can maintain monocultures within a HRFAP system. Once implemented at scale, optimal operational parameters can be determined to further increase biomass productivities and bioremediation performance of target cultivars.
Data availability
All data generated or analysed during this research isavailable from the corresponding author upon reasonable demand.
References
Arzate S, Pfister S, Oberschelp C, Sánchez-Pérez JA (2019) Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci Total Environ 694:133572
Baghour M (2019) Algal degradation of organic pollutants. In: Martínez LMT, Kharissova OV, Kharisov BI (eds) Handbook of Ecomaterials. Springer, Cham, pp 565–586
Bao B, Thomas-Hall SR, Schenk PM (2022) Fast-Tracking isolation, identification and characterization of new microalgae for nutraceutical and feed applications. Phycology 2:86–108
Bellinger EG, Sigee DC, Bellinger DE, Sigee DDD (2010) Freshwater Algae : Identification and Use As Bioindicators. John Wiley & Sons, Hoboken
Bhatt A, Agrawal K, Verma P (2021) Phycoremediation: Treatment of pollutants and an initiative towards sustainable environment. In: Prasad R (ed) Phytoremediation for Environmental Sustainability. Springer, Singapore, pp 485–511
Borchhardt N, Gründling-Pfaff S (2020) Ecophysiological response against temperature in Klebsormidium (Streptophyta) strains isolated from biological soil crusts of Arctic and Antarctica indicate survival during global warming. Front Ecol Evol 8:153
Borowitzka MA (2013) Species and strain selection. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 77–89
Brawley SH, Johnson LE (1992) Gametogenesis, gametes and zygotes: an ecological perspective on sexual reproduction in the algae. Br Phycol J 27:233–252
Carey RO, Migliaccio KW (2009) Contribution of wastewater treatment plant effluents to nutrient dynamics in aquatic systems: a review. Environ Manage 44:205–217
Catone CM, Ripa M, Geremia E, Ulgiati S (2021) Bio-products from algae-based biorefinery on wastewater: a review. J Environ Manage 293:112792
Champion PD (2018) Knowledge to action on aquatic invasive species: island biosecurity–the New Zealand and South Pacific story. Manage Biol Invas 9:383–394
Cheregi O, Ekendahl S, Engelbrektsson J, Strömberg N, Godhe A, Spetea C (2019) Microalgae biotechnology in Nordic countries - the potential of local strains. Physiol Plant 166:438–450
Chrispim MC, Scholz M, Nolasco MA (2019) Phosphorus recovery from municipal wastewater treatment: critical review of challenges and opportunities for developing countries. J Environ Manage 248:109268
Cole A, Dinburg Y, Haynes BS, He Y, Herskowitz M, Jazrawi C, Landau M, Liang X, Magnusson M, Maschmeyer T (2016) From macroalgae to liquid fuel via waste-water remediation, hydrothermal upgrading, carbon dioxide hydrogenation and hydrotreating. Energy Environ Sci 9:1828–1840
Cole A, Praeger C, Mannering T, de Nys R, Magnusson M (2018) Hot and bright: thermal and light environments for the culture of Oedogonium intermedium and the geographical limits for large-scale cultivation in Australia. Algal Res 34:209–216
Craggs R, Park J, Heubeck S, Sutherland D (2014) High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. N Z J Bot 52:60–73
Cruz H, Law YY, Guest JS, Rabaey K, Batstone D, Laycock B, Verstraete W, Pikaar I (2019) Mainstream ammonium recovery to advance sustainable urban wastewater management. Environ Sci Technol 53:11066–11079
De La Bueno Cueva P, Gillerman L, Gehr R, Oron G (2017) Nanotechnology for sustainable wastewater treatment and use for agricultural production: a comparative long-term study. Water Res 110:66–73
de Paula Silva PH, De Nys R, Paul NA (2012) Seasonal growth dynamics and resilience of the green tide alga Cladophora coelothrix in high-nutrient tropical aquaculture. Aquacult Environ Interact 2:253–266
Divya, Dasauni K, Nailwal T (2023) Addressing the strategies of algal biomass production with wastewater treatment. In: Shah MP (ed) Phycoremediation Processes in Industrial Wastewater Treatment. CRC Press, Boca Raton, pp 1–20
Figueroa FL, Jerez CG, Korbee N (2013) Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Latin Am J Aquat Res 41:801–819
Garfí M, Flores L, Ferrer I (2017) Life Cycle Assessment of wastewater treatment systems for small communities: activated sludge, constructed wetlands and high rate algal ponds. J Cleaner Produc 161:211–219
Ge S, Champagne P (2017) Cultivation of the marine macroalgae Chaetomorpha linum in municipal wastewater for nutrient recovery and biomass production. Env Sci Technol 51:3558–3566
Ge S, Madill M, Champagne P (2018) Use of freshwater macroalgae Spirogyra sp. for the treatment of municipal wastewaters and biomass production for biofuel applications. Biomass Bioenergy 111:213–223
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Hariz HB, Lawton RJ, Craggs RJ (2022) Novel assay for attached filamentous algae productivity and nutrient removal. J Appl Phycol 25:251–264
Hariz HB, Lawton RJ, Craggs RJ (2023) Nutrient uptake and biomass productivity performance comparison among freshwater filamentous algae species on mesocosm-scale FANS under ambient summer and winter conditions. Ecol Eng 189:106910
John DM, Whitton BA, Brook AJ (2011) The freshwater algal flora of the British Isles : an identification guide to freshwater and terrestrial algae, 2nd edn. Cambridge University Press, Cambridge
Karri RR, Sahu JN, Chimmiri V (2018) Critical review of abatement of ammonia from wastewater. J Molec Liquids 261:21–31
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241
Kebede-Westhead E, Pizarro C, Mulbry W (2006) Treatment of swine manure effluent using freshwater algae: Production, nutrient recovery, and elemental composition of algal biomass at four effluent loading rates. J Appl Phycol 18:41–46
Kehrein P, van Loosdrecht M, Osseweijer P, Garfí M, Dewulf J, Posada J (2020) A critical review of resource recovery from municipal wastewater treatment plants – market supply potentials, technologies and bottlenecks. Environ Sci: Water Res Technol 6:877–910
Krause-Jensen D, Carstensen J, Dahl K (2007) Total and opportunistic algal cover in relation to environmental variables. Mar Pollut Bull 55:114–125
Kromkamp JC, Dijkman NA, Peene J, Simis SG, Gons HJ (2008) Estimating phytoplankton primary production in Lake IJsselmeer (The Netherlands) using variable fluorescence (PAM-FRRF) and C-uptake techniques. Eur J Phycol 43:327–344
Kube M, Jefferson B, Fan L, Roddick F (2018) The impact of wastewater characteristics, algal species selection and immobilisation on simultaneous nitrogen and phosphorus removal. Algal Res 31:478–488
Lam MK, Lee KT (2014) Scale-up and commercialization of algal cultivation and biofuel production. In: Pandey A, Lee D-J, Chisti Y, Soccol CR (eds) Biofuels from Algae. Elsevier, Amsterdam, pp 261–286
Lavrinovičs A, Juhna T (2017) Review on challenges and limitations for algae-based wastewater treatment. Construct Sci 20:17–25
Lawton RJ, de Nys R, Paul NA (2013) Selecting reliable and robust freshwater macroalgae for biomass applications. PLoS One 8(5):e64168
Lawton RJ, de Nys R, Skinner S, Paul NA (2014) Isolation and identification of Oedogonium species and strains for biomass applications. PLoS One 9(3):e90223
Lawton RJ, Cole AJ, Roberts DA, Paul NA, de Nys R (2017) The industrial ecology of freshwater macroalgae for biomass applications. Algal Res 24:486–491
Lawton RJ, Glasson CR, Novis PM, Sutherland JE, Magnusson ME (2021) Productivity and municipal wastewater nutrient bioremediation performance of new filamentous green macroalgal cultivars. J Appl Phycol 33:4137–4148
Lawton RJ, Sutherland JE, Glasson CR, Magnusson ME (2021) Selection of temperate Ulva species and cultivars for land-based cultivation and biomass applications. Algal Res 56:102320
Lehtovirta-Morley LE (2018) Ammonia oxidation: Ecology, physiology, biochemistry and why they must all come together. FEMS Microbiol Lett 365:fny058
Leong YK, Huang C-Y, Chang J-S (2021) Pollution prevention and waste phycoremediation by algal-based wastewater treatment technologies: the applications of high-rate algal ponds (HRAPs) and algal turf scrubber (ATS). J Environ Manage 296:113193
Li Y, Wood E, Kosa G, Muzamil B, Vogelsang C, Holmstad R (2021) A new insight of phycoremediation study: Using filamentous Algae for the treatment of tertiary municipal wastewater. In: Zepka LQ, Jacob-LOpez E, Deprá MC (eds) Progress in Microalgae Research - A path for Shaping Sustainable Futures. IntechOpen, Rijeka, pp 147–164
Liu J, Vyverman W (2015) Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresour Technol 179:234–242
Liu J, Danneels B, Vanormelingen P, Vyverman W (2016) Nutrient removal from horticultural wastewater by benthic filamentous algae Klebsormidium sp., Stigeoclonium spp. and their communities: From laboratory flask to outdoor Algal Turf Scrubber (ATS). Water Res 92:61–68
Liu J, Pemberton B, Lewis J, Scales PJ, Martin GJO (2020) Wastewater treatment using filamentous algae – a review. Bioresour Technol 298:122556
Lu Q, Chen P, Addy M, Zhang R, Deng X, Ma Y, Cheng Y, Hussain F, Chen C, Liu Y, Ruan R (2018) Carbon-dependent alleviation of ammonia toxicity for algae cultivation and associated mechanisms exploration. Bioresour Technol 249:99–107
Neveux N, Magnusson M, Mata L, Whelan A, de Nys R, Paul NA (2016) The treatment of municipal wastewater by the macroalga Oedogonium sp. and its potential for the production of biocrude. Algal Res 13:284–292
Nguyen LN, Aditya L, Vu HP, Johir AH, Bennar L, Ralph P, Hoang NB, Zdarta J, Nghiem LD (2022) Nutrient removal by algae-based wastewater treatment. Curr Pollut Rep 8:369–383
Pankratz S, Oyedun AO, Kumar A (2019) Development of cost models of algae Production in a cold climate using different production systems. Biofuels, Biofuels Bioprod Bioref 13:1246–1260
Park J, Craggs R (2011) Nutrient removal in wastewater treatment high rate algal ponds with carbon dioxide addition. Water Sci technol 63:1758–1764
Pikosz M, Messyasz B, Gąbka M (2017) Functional structure of algal mat (Cladophora glomerata) in a freshwater in western Poland. Ecol Indicat 74:1–9
Rani S, Gunjyal N, Ojha CSP, Singh Rajendra P (2021) Review of challenges for algae-based wastewater treatment: strain selection, wastewater characteristics, abiotic, and biotic factors. J Hazard Toxic Radioact Waste 25:03120004
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873
Renuka N, Sood A, Ratha SK, Prasanna R, Ahluwalia AS (2013) Nutrient sequestration, biomass production by microalgae and phytoremediation of sewage water. Int J Phytoremed 15:789–800
Ricciardi A, Simberloff D (2009) Assisted colonization is not a viable conservation strategy. Trends Ecol Evol 24:248–253
Robinson N, Winberg P, Kirkendale L (2013) Genetic improvement of macroalgae: status to date and needs for the future. J Appl Phycol 25:703–716
Ross M, Davis K, McColl R, Stanley MS, Day JG, Semião AJC (2018) Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: Influence on growth, nitrogen preference and biochemical composition. Algal Res 30:1–10
Ryther J, Guillard R (1962) Studies of marine planktonic diatoms: III. Some effects of temperature on respiration of five species. Can J Microbiol 8:447–453
Salbitani G, Carfagna S (2021) Ammonium utilization in microalgae: A sustainable method for wastewater treatment. Sustainability 13:956
Salgot M, Folch M (2018) Wastewater treatment and water reuse. Curr Opin Environ Sci Health 2:64–74
Sameena PP, Janeeshma E, Sarath NG, Puthur JT (2022) Phytoremediation and phycoremediation: A sustainable solution for wastewater treatment. In: Madhav S, Singh P, Mishra V, Ahmed S, Mishra PK (eds) Recent Trends in Wastewater Treatment. Springer, Cham, pp 171–191
Silkina A, Nelson GD, Bayliss CE, Pooley CL, Day JG (2017) Bioremediation efficacy—comparison of nutrient removal from an anaerobic digest waste-based medium by an algal consortium before and after cryopreservation. J Appl Phycol 29:1331–1341
Simha P, Ganesapillai M (2017) Ecological Sanitation and nutrient recovery from human urine: how far have we come? a review. Sustain Environ Res 27:107–116
Škufca D, Kovačič A, Prosenc F, Griessler Bulc T, Heath D, Heath E (2021) Phycoremediation of municipal wastewater: removal of nutrients and contaminants of emerging concern. Sci Total Environ 782:146949
Stachowicz JJ, Graham M, Bracken MES, Szoboszlai AI (2008) Diversity enhances cover and stability of seaweed assemblages: the role of heterogeneity and time. Ecology 89:3008–3019
Stirbet A (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B 104:236–257
Sutherland DL, Ralph PJ (2020) 15 years of research on wastewater treatment high rate algal ponds in New Zealand: discoveries and future directions. N Z J Bot 58:334–357
Sutherland DL, Howard-Williams C, Turnbull MH, Broady PA, Craggs RJ (2015) The effects of CO2 addition along a pH gradient on wastewater microalgal photo-physiology, biomass production and nutrient removal. Water Res 70:9–26
Tchobanoglous G, Burton FL, Stensel HD (2003) Wastewater engineering : treatment and reuse, 4th edn. McGraw-Hill, New York
Vadeboncoeur Y, Moore MV, Stewart SD, Chandra S, Atkins KS, Baron JS, Bouma-Gregson K, Brothers S, Francoeur SN, Genzoli L, Higgins SN, Hilt S, Katona LR, Kelly D, Oleksy IA, Ozersky T, Power ME, Roberts D, Smits AP, Timoshkin O, Tromboni F, Zanden MJV, Volkova EA, Waters S, Wood SA, Yamamuro M (2021) Blue waters, green bottoms: Benthic filamentous algal blooms are an emerging threat to clear lakes worldwide. BioScience 71:1011–1027
Valero-Rodriguez JM, Swearer SE, Dempster T, de Nys R, Cole AJ (2020) Evaluating the performance of freshwater macroalgae in the bioremediation of nutrient-enriched water in temperate environments. J Appl Phycol 32:641–652
van Puijenbroek PJTM, Beusen AHW, Bouwman AF (2019) Global nitrogen and phosphorus in urban waste water based on the shared socio-economic pathways. J Environ Manage 231:446–456
Wang H, Hu Z, Xiao B, Cheng Q, Li F (2013) Ammonium nitrogen removal in batch cultures treating digested piggery wastewater with microalgae Oedogonium sp. Water Sci Technol 68:269–275
Wreford A, Bayne K, Edwards P, Renwick A (2019) Enabling a transformation to a bioeconomy in New Zealand. Environ Innovat Societal Transit 31:184–199
Young P, Taylor M, Fallowfield HJ (2017) Mini-review: high rate algal ponds, flexible systems for sustainable wastewater treatment. World J Microbiol Biotechnol 33:117
Yun J-H, Smith VH, deNoyelles FJ, Roberts GW, Stagg-Williams SM (2014) Freshwater macroalgae as a biofuels feedstock: mini-review and assessment of their bioenergy potential. Indust Biotechnol 10:212–220
Zhou Y, Meng J, Zhang M, Chen S, He B, Zhao H, Li Q, Zhang S, Wang T (2019) Which type of pollutants need to be controlled with priority in wastewater treatment plants: traditional or emerging pollutants? Environ Internat 131:104982
Acknowledgements
The authors would like to thank Harizah Hariz, Denise Rendle, Peter Randrup and Ari Brandenburg for their assistance with experiments and Western Bay of Plenty District Council for allowing wastewater and algae collection from their wastewater treatment plant.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was funded by the New Zealand National Institute of Water and Atmospheric Research through a Ministry of Business, Innovation and Employment endeavour research programme contract number C01X1912.
Author information
Authors and Affiliations
Contributions
Indira N. Novak: Methodology, Investigation, Data curation, Formal analysis, Writing - original draft.
Rebecca J. Lawton: Supervision, Conceptualization, Writing – review and editing.
Marie Magnusson: Supervision, Conceptualization, Writing - review and editing.
Rupert J. Craggs: Supervision, Conceptualization, Writing – review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
All authors declare there is no conflict of interest. The authors report no commercial or proprietary interest in any product or concept discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Novak, I.N., Magnusson, M., Craggs, R.J. et al. Screening protocol for freshwater filamentous macroalgae bioremediation of primary municipal wastewater. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03261-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03261-7