Abstract
The bioavailability of Arthrospira platensis as an antioxidant in fish oil after fermentation and microencapsulation process was investigated in this study. Unfermented and fermented A. platensis (Spirulina) were dried in a spray dryer with different ratios of maltodextrin and the powdered Spirulina samples were added to fish oil at a rate of 2% to monitor oxidative stability. Following the BHT (butylated hydroxytoluene) added group, unfermented and fermented Spirulina samples and those coated with equal amounts of maltodextrin showed the lowest peroxide values and thiobarbituric acid reactive substance values. On the other hand, according to p-anisidine and Totox (total oxidation) assessments, all Spirulina-added groups were at least as effective as BHT in preventing lipid oxidation. At the end of storage, total polyunsaturated fatty acid (PUFA) content was determined as 21.09% in the control group without antioxidants and 28.75% in the group with BHT added. However, in all other Spirulina added groups (unfermented, fermented and microencapsulated groups), total PUFA content was in the range of 27.671-29.72%. As a result, it was found that both the dried forms of Spirulina alone and dried with coating material were effective in delaying lipid oxidation. However, the fermented forms showed no additional effect on lipid stability compared to the unfermented ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional oils have health-promoting and disease-preventing properties such as improving lipid and glucose metabolism, antithrombosis and improving cognitive ability, preventing cardiovascular diseases, neurological disorders and even certain types of cancer (Day et al. 2009; Gerber 2012; Ajith and Jayakumar 2019). Due to these health benefits, marine and plant oils containing essential fatty acids and minor bioactive components, for example fish, algae, seed, nut, and fruit oils, are considered functional oils (Shabbir et al. 2023). Therefore, the demands for functional oils in food, pharmaceutical and cosmetic industries are constantly increasing. However, ω-3 PUFA-rich functional oils are very sensitive to oxidation, resulting in an unpleasant taste from spoilage, limited shelf life and some safety issues. Because of their sensitivity to light and heat, functional oils are unstable under processing and storage conditions and must be preserved to improve their stability during transport, processing and storage. The stability of oils can be improved by reducing the activity of oxidation-inducing enzymes, using chelating agents and choosing appropriate packaging methods. Antioxidants, especially natural antioxidants, are another crucial tool. The use of synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and tert-butylhydroquinone (TBHQ) has limitations due to toxicological concerns raised as a result of their long-term use. Kozłowska and Gruczyńska (2018) added different concentrations of marjoram, thyme and oregano extracts to edible oils and compared them with synthetic antioxidant (BHT). The researchers stated that sunflower oil with oregano extract and soybean oil with thyme extract had better oxidative stability than the synthetic or non-antioxidant groups. Alavi and Golmakani (2017) determined that Spirulina at different concentrations increased the oxidative stability of olive oil under accelerated storage and was more effective than α-tocopherol.
The cyanobacterium Arthrospira platensis (Spirulina) has antioxidant activity due to its carotenoid, chlorophyll and phenolic components, and antidiabetic, anticancer, immune system strengthening and antibiotic properties are also reported (Mendiola et al. 2007). In addition, Spirulina is an excellent source of phycocyanin. Phycocyanin is known to have anti-inflammatory, antioxidative, hepatoprotective and radical scavenging properties (Romay et al. 2003). Furthermore, phycocyanin has demonstrated strong therapeutic potential in the treatment of cancer and immunomodulation (Wu et al. 2016). Spirulina can be regarded as a crucial ingredient for the production of functional foods because of its high protein content and bioactive components. Furthermore, recently, the fermented forms of Spirulina have also got some attention due to the improvements of their bioactive contents and increased bioefficiency. However, in both forms, the main obstacles in the use of Spirulina in foods are their high moisture contents and its self-sensitive properties. In general, difficulties occur while adding Spirulina to food because of their unpleasant aroma and dark green color. The most convenient way to overcome these obstacles is through the microencapsulation of Spirulina. Because the wall material used in microencapsulation can mask the sensory properties of Spirulina, preserve its bioactive components and by removing moisture improve its addition to food, transportation and storage. Özyurt et al. (2023) investigated the nutritional components and changes in color and morphological appearance of unfermented and fermented Spirulina microencapsulated with different ratios of coating materials. The researchers stated that Spirulina fermented with Lactobacillus plantarum increased total organic acid and lysine contents, but no improvement in other nutrient components. It was also determined that fermented Spirulina were coated more effectively with wall material. Kuley et al. (2023) also reported that the drying and fermentation processes in Spirulina significantly increased the release of total phenol content, total antioxidant activity, DPPH free radical scavenging capacity, and antimicrobial activity. The aim of this study was to determine the effects of microcapsule forms of fermented and unfermented Spirulina on the oxidative stability of fish oil, which is functional oil. The findings highlight the potential for the application of the microcapsule form of unfermented and fermented Spirulina in the food industry and can provide a basis for the production of functional foods based on Spirulina.
Materials and methods
Preparation of Spirulina samples
Arthrospira platensis grown under laboratory conditions at the Çukurova University Algal Biotechnology Unit was used. At the harvesting stage, three different sizes of porous cloth (200, 100 and 40 μm) were used to filter the culture. Concentration was achieved by washing the wet Spirulina biomass accumulated at 100 and 40 μm with distilled water. The wet biomass harvested for the experiment was kept in storage at +4°C. For Spirulina fermentation, Lactobacillus plantarum FI 8595 was obtained from the Department of Food Engineering of Sütçü İmam Kahramanmaraş University (Turkey). The L. plantarum strain was activated in MRS broth (MRS, Merck 1.10661, Germany) at 37 °C before use.
Spirulina was fermented with L. plantarum according to Özyurt et al (2023). Spirulina with 10% dry weight was inoculated with 5% (v/v) L. plantarum at a concentration of 2 McFarland (6.0x108 cfu mL-1). Following the addition of glucose (2%, w/v) and potassium sorbate (0.2%, w/v), the mixture was incubated anaerobically for 72 h at 37 °C. In order to stop the fermentation, an ultraviolet treatment was performed for 20 min. Fermentation processes were performed in triplicate.
After fermentation, unfermented and fermented wet Spirulina biomass were dried with a spray dryer (B-290, Buchi-Mini Spray Dryer, Switzerland). Maltodextrin (dextrose equivalent: 18-20) was used as a coating material for unfermented and fermented Spirulina. The emulsion's total solids concentration was adjusted to 30%. The ratios of Spirulina to coating material (Spirulina: maltodextrin, w/w) were 1:0, 1:1, and 1:2. The aspiration rate was set to about 30 m3 h-1, and the emulsion feed flow rate was adjusted to 20 mL min-1 in the spray drying system. The inlet temperature was set to 170 °C and the outlet temperature was recorded as 95 ± 5 °C. Powdered, unfermented and fermented Spirulina samples were collected from the cyclone separator and kept in airtight dark glass bottles at 2 °C for further analysis. Dried Spirulina samples were coded as shown in Fig. 1.
Blending Spirulina with fish oil and accelerated oxidation during storage
Unfermented and fermented Spirulina were dried in spray dryer with different ratios of coating material, and the powdered Spirulina samples were added to fish oil at the concentration of 2% and mixed thoroughly to ensure homogeneity (Alavi and Golmakani 2017). For comparison, in addition to the control group without Spirulina, another group with 200 ppm BHT was also prepared. All groups were placed in glass jars with at least three parallels and kept in an incubator set at 60 °C for 14 days, and their oxidative stability was assessed using the accelerated storage method. Fish oil samples taken randomly on days 0, 3, 6, 9 and 14 were evaluated for oxidative stability by the following analyses. The experimental design of the research is shown in Fig. 1.
Free Fatty Acids (FFA)
The Cd5a-40 procedure (AOCS 1994) was used to assess the free fatty acid (FFA) content of fish oils. Free fatty acids were measured as a percentage of oleic acid using an acidimetric titration after the addition of ethanol and the indicator phenolphthalein. The following equation was used to calculate the FAA content:
Peroxide Value (PV)
The Cd8-53 procedure (AOCS 1994) was used to measure the peroxide values (PV) of fish oil groups. The oil sample was weighed and combined with a solvent solution made up of 60% acetic acid and 40% chloroform (v/v). Then, saturated potassium iodide (KI) solution was added to this mixture. By using starch as an indication, liberated iodine was titrated with 0.002 M sodium thiosulfate. The results were reported as milliequivalents of peroxide oxygen per kilogram of oil. The following equations was used to calculate PV:
where B = titration of blank, S = titration of sample, N = normality of sodium thiosulfate solution.
Thiobarbituric Acid Reactive Substance (TBARs) Value
AOCS method Cd 19–90 (AOCS 1998) was used to measure the thiobarbituric acid reactive substance (TBARs) values of fish oil groups. Fish oil samples were dissolved in 1-butanol, combined with TBA reagent (0.02% in 1-butanol) and then incubated for 2 h at 95 °C in a water bath. The absorbance of the samples was determined at 532 nm. The results are presented in mg MDA kg-1 fish oil.
p-Anisidine Value (pAV) and Totox Value
The value of p-anisidine (pAV) was measured using IUPAC method 2.504 ((IUPAC 1987). The process relies on the spectrophotometric analysis of the products formed by the reaction of p-anisidine with aldehydes in oil. The following formula (3) was used to determine the value of p-anisidine:
where As = the absorbance of the fat solution after reaction with the p-anisidine reagent, Ab = the absorbance of the fat solution, m = the mass of the fish oil (g).
The Totox value was calculated by the following Eq. (4):
Fatty acid composition
Fish oils were pipetted into a tube and fragmented into their component FAME (fatty acid methyl esters) according to the method of Ichihara et al. (1996). n-heptane (2 mL) and 2 M methanolic KOH (4 mL) were used to dissolve the sample (10 mg). After the mixture was centrifuged at 4000 rpm for 10 min, the n-heptane layer was separated for GC analyses.
The procedures were carried out using an Agilent GC-MS/MS (7890B, GC-7010B MS, Germany) equipped with a flame ionization detector and a capillary Agilent J&W DB-WAX column (60m x 0.25 µm x 0.25µm). The oven temperature was programmed at 50°C for 1 min and then increased to 200°C at a rate of 25°C min-1. It was raised to 230 °C after 10 min at a rate of 3°C min-1, and this temperature was maintained for 18 min. The injector and detector were programmed to operate at 250°C and 300°C, respectively. The sample size was set to 1 µL and flow rate of the carrier gas was determined as 1 mL min-1. The split ratio was set to 1:40. The FAME containing 37 standard components (Supelco-37, USA) was used for the identification of fatty acids.
Statistical analyses
Data were analysed according to one-way analysis of variance (ANOVA) at a 5% level of confidence using Duncan's multiple range test.
Results and discussion
Lipid stability of fish oil with the addition of unfermented and fermented Spirulina
Lipid stability is an important criterion for fish oil for maintaining its functional food properties. The oxidation of lipids can cause significant loss of sensory quality and lead to the emergence of many diseases through oxidation end-products. The results of free fatty acids (FFA), peroxide (PV), thiobarbituric acid reactive substances (TBARs), p-anisidine (pAV) and totox measurements performed to evaluate the lipid quality of fish oil under accelerated storage conditions (60°C) are shown in Fig. 2 (a-e). Fish oil typically contains high levels of free fatty acid (FFA) contents because of their high autolytic activity and polyunsaturated fatty acid content, which makes them highly susceptible to lipolysis and oxidation. The formation of FFA does not result in nutritional losses, but an increase in lipid oxidation products can be observed. At the beginning of storage, the FFA value of raw fish oil was found to be 2.06±0.33% oleic acid. Similarly, EFSA (EFSA Panel on Biological Hazards (BIOHAZ) 2010) reported that the FFA content in raw fish generally in the range of 2-5% oleic acid. It was observed that the FFA content increased significantly in all groups throughout the storage period at 60°C (P<0.05). During the storage period, a gradual increase in FFA content in the control group was observed. However, the FFA content remained at the same level until day 14 in the fish oil group containing BHT. On the other hand, although low FFA values were observed in the first 6 days in the fish oil groups containing unfermented and fermented Spirulina, no statistically significant difference was observed from the control or BHT added fish oil groups (Fig 2a).
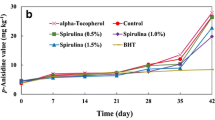
Peroxide value (PV), an important indicator of the early stages of oxidation, is frequently used for assessing the overall hydroperoxide content and monitors the progress of lipid oxidation throughout oil processing and preservation. The PV of all samples subjected to heat oxidation during the storage period is shown in Fig. 2b. It was determined that the peroxide value of raw fish oil, which was measured as 1.45±0.07 at the beginning of storage, increased significantly during the storage period in all stored groups (P<0.05). The highest PVs were observed in the control group during the accelerated oxidation process (P<0.05). However, the PVs of BHT, spray dried unfermented and fermented Spirulina added fish oil groups were significantly lower than the control group. On the 9th day, it was recorded that the PVs of all Spirulina added groups were similar to that of the synthetic antioxidant added (FO-BHT) group (P>0.05). On the 14th day, the lowest PVs were determined in FO-USp, FO-USp/1MD, FO-FSp, FO-FSp/1MD and FO-FSp/2MD groups after the BHT added group. Therefore, it can be concluded that the addition of spray dried unfermented and fermented Spirulina was comparable to BHT in delaying the formation of primary oxidation products in fish oil. Similarly, Alavi and Golmakani (2017) reported that adding 0.5, 1 or 1.5% Spirulina to olive oil resulted in lower peroxide levels than adding alpha tocopherol. The researchers also recorded similar PVs with the BHT added group until the 35th day of storage, but higher PVs were recorded for the Spirulina group on the 42nd day. In this study, it was observed that Spirulina spray-dried with 1:1 and 1:2 (Spirulina:MD) showed a similar effect to the spray-dried without MD in delaying the oxidation of fish oil. Accordingly, it can be thought that spray drying with MD allows controlled release of the antioxidant activity of Spirulina. However, it was observed that fermented forms did not make a significant difference.
The basis of TBARs analysis is that coloured compounds are formed when TBA reacts with malonaldehyde or other TBA-reactive chemicals from oxidized lipids. In this study, the TBA value of raw fish oil was determined as 0.33±0.00 mg MA kg-1, and significant increases were observed in all groups during the storage period (Fig 2c). While TBARs values of all groups were similar on the 6th day of storage (P>0.05), the lowest value was determined in FO-BHT group and the highest value was determined in FO-C group on the 9th day of storage (P<0.05). However, the lowest TBARs values on the 14th day of storage were determined in FO-USp (6.42 mg MA kg-1), FO-FSp (6.52 mg MA kg-1), FO-USp/1MD (6.80 mg MA kg-1), FO-BHT (7.10 mg MA kg-1) and FO-FSp-1MD (7.50 mg MA kg-1) groups. Therefore, we can conclude that unfermented and fermented Spirulina and/or samples coated with MD in equal proportions were as effective as BHT additive. However, groups coated with 2-fold MD of Spirulina (FO-USp/2MD and FO-FSp/2MD) were not found to be effective in terms of TBARs evaluation. Similarly, Takyar et al. (2019) evaluated the antioxidant effect of alcoholic extracts of Chlorella vulgaris and A. platensis in extending the shelf life of rainbow trout (Oncorhynchus mykiss) fillet at 4 ± 1 °C. They found that Spirulina extract was more effective in providing a low TBA value. Rosas et al. (2019) also found that mullet (Mugil liza) fillets fed by Spirulina supplementation showed lower lipid peroxidation (TBARs) than those fed by pure β-carotene supplementation. The researchers reported that Spirulina had a greater capacity for inhibiting the lipid peroxidation and that Spirulina was a more suitable additive for fish diets than pure β-carotene.
P-Anisidine is also used as a good indicator to monitor secondary oxidation products formed during oxidation. At the beginning of storage, pAV was measured as 10.16±1.91 in raw fish oil and the highest values were found in the control group throughout the storage (Fig 2d). After the 6th day of storage, the lowest values were observed in FO-USp and FO-FSp groups. These groups were unfermented and fermented Spirulina samples dried in spray dryer without the use of a carrier agent. The addition of these samples to fish oil appears to be as effective as the addition of BHT during the whole storage period. However, it is also remarkable that on the last day of storage, all Spirulina added groups had significantly lower pAV values than BHT added groups (P>0.05). Similarly, Kahraman and Özdemir (2021) investigated the effects of Spirulina and black elderberry extracts on the oxidative stability of cold-pressed flaxseed oil under accelerated storage conditions. They reported that Spirulina extract was more effective on pAV values than black elderberry extract and synthetic antioxidant (BHT). Totox values, which evaluate total oxidation, similarly showed that FO-USp and FO-FSp were the best groups during the storage process. In addition, it was observed that the totox contents of all other groups containing Spirulina were similar to the group containing BHT (Fig. 2e). Kahraman and Özdemir (2021) found that the addition of ethanol extracts of black elderberry and Spirulina to linseed oil provided better oxidative stability than BHT and gallic acid. Similarly, Özogul et al. (2021) stated that Spirulina extract delayed the lipid oxidation during cold storage of sardine meat. In our previous study, it was determined that the fermented form of Spirulina had higher total phenol content and DPPH activity (Kuley et al. 2023). Kuley et al. (2023) also reported that the encapsulation of Spirulina with maltodextrin at a ratio of 1:1 gave better results than the 1:2 ratios in terms of DPPH. Similar results were recorded in total antioxidant values. In many studies, it has been reported that lactic acid fermentation has higher total phenolic content and DPPH radical scavenging activity compared to unfermented Spirulina (Liu et al. 2011; Jamnik et al. 2022; Pérez-Alva et al. 2022). On the other hand, there are studies indicating that there is little or no difference in antioxidant capacity between fermented and unfermented seaweed (Takei et al. 2017; Niccolai et al. 2020).
As a result, it was observed that the dried forms of Spirulina alone and dried with a coating material were effective in delaying lipid oxidation. However, no differentiation was observed in the fermented forms compared to the unfermented forms in terms of maintaining lipid stability. Since it was observed that Spirulina used in microencapsulated form was as effective as single dried forms, it can be said that this process has the potential to lead more efficient use of Spirulina.
Fatty acid composition of fish oil with the addition of unfermented and fermented Spirulina
The most important factor affecting the oxidative susceptibility of lipids is the composition of fatty acids, specifically their degree of unsaturation or methylene bridge index (Shahidi and Zhong 2010). According to deMan (1992), the oxidation rates for C18:0, C18:1, C18:2 and C18:3 are 1:100:1200:2500 at 100°C, respectively. Therefore, fish oils rich in PUFAs with high methylene bridge index values are highly susceptible to oxidation. An additional indicator of the degree of oxidation is the compositional changes that occur in especially unsaturated fatty acids during oxidation (Shahidi and Zhong 2010). In this study, the changes of fatty acid composition of fish oil under accelerated storage conditions (60°C) are shown in Table (1). The main fatty acids determined in raw fish oil were palmitic acid, oleic acid, docosahexaenoic acid (DHA, C22:6ω3), eicosapentaenoic acid (EPA, C20:5ω3), myristic acid, palmitoleic acid, and stearic acid. These findings were generally consistent with those published for anchovy oil (Yeşilsu and Özyurt 2019; Özyurt et al. 2022).
It was observed that there was no significant change in the content of palmitic acid, the highest saturated fatty acid in all groups, during the storage period. However, the fluctuations determined in myristic and stearic fatty acids were also reflected in the total saturated fatty acids (SFA) ratio (Fig. 3). Oleic acid and palmitoleic fatty acids were the predominant monounsaturated fatty acids (MUFA) in all groups during storage. Significant decreases in the ratio of these fatty acids were observed only in the group without antioxidant addition (FO-C) (P<0.05).
The most important properties that distinguish fish oils from all other oils are their high PUFA content, especially EPA and DHA. The long-chain unsaturated fatty acid content of most vegetable oils is rarely 5% and usually around 1%. In this study, it was determined that PUFAs constituted approximately 30% of the total fatty acids of fish oil at the beginning of storage. This composition consisted of approximately 50% DHA and 30% EPA concentration. As a result of storage at 60°C, the highest decrease in EPA and DHA ratio was detected in the control group without antioxidant addition (P<0.05). It was found that EPA and DHA ratios in the other groups were similar to the BHT supplemented group (P>0.05). In the control group, the decrease in EPA, DHA, oleic acid and palmitoleic acid ratios was parallel to the increase in stearic acid ratio and the detection of higher oxidation products. At the end of storage, the total PUFA content of the BHT-added group was found to be 28.75%, compared to 21.09% for the control group without antioxidant. However, the total PUFA content was in the range of 27.671-29.72% in all Spirulina-added groups (FO-USp, FO-USp/1MD, FO-USp/2MD, FO-FSp, FO-FSp/1MD, FO-FSp-2MD). It can be concluded that inclusion of spray dried unfermented and fermented Spirulina in fish oil significantly suppressed the reduction of ΣMUFA and Σ PUFA.
Golmakani et al (2018) reported that kilka (Clupeonella cultriventris caspia) oil containing Spirulina extract showed significantly lower Totox, peroxide and p-anisidine values compared to the control group during the 15 days storage at 60 °C. Morsy et al. (2019) added whole Spirulina cells and Spirulina nanoparticles to olive oil and compared them with BHT and alpha tocopherol in addition to the control group. The researchers reported that the oxidation parameters of olive oil containing Spirulina nanoparticles were lower than the control. However, since fatty acid profiles were not examined in these studies, a comparison could not be made. Similarly, this study revealed that unfermented and fermented Spirulina and their microcapsule forms were effective in delaying the fish oil oxidation and also preserved fatty acid profiles.
Conclusion
Health benefits from fish oils, which are the most important source of EPA and DHA known in nature, depend on the protection of these fatty acids during processing and storage. In this study, the most dramatic reduction in total PUFA relative to the EPA and DHA content of fish oil was observed in the control group without antioxidant. In general, FO-USp and FO-FSp were the most effective groups in terms of oxidation parameters and all other Spirulina added groups were found to be as effective as BHT in preventing lipid oxidation. Spirulina is a valuable food source of antioxidants proven by many studies. The enrichment of Spirulina's bioactive characteristics by fermentation is also one of the significant topics in recent years. In this study, the effectiveness of microencapsulation of unfermented or fermented Spirulina, which facilitates its inclusion in the food system by preserving its valuable properties, was observed in fish oil. It can be stated that Spirulina's potential for consumption with other foods will be increased with microencapsulation which is one of the most practical techniques for the addition, transportation, and storage of Spirulina to other foods in the industry.
Data availability
The corresponding author can provide the datasets upon reasonable request.
References
Ajith TA, Jayakumar TG (2019) Omega-3 fatty acids in coronary heart disease: Recent updates and future perspectives. Clin Exp Pharmacol Physiol 46:11–18
Alavi N, Golmakani M-T (2017) Antioxidant properties of whole-cell Spirulina (Arthrospira platensis) powder expressed in olive oil under accelerated storage conditions. J Appl Phycol 29:2971–2978
AOCS (1994) AOCS Official Method Cd 8–53 and Cd 5a-40. Official methods and recommended practices of the American Oil Chemists Society. American Oil Chemistry Society, Champaign, IL
AOCS (1998) Official method Cd 19–90. 2-Thiobarbituric acid value. Direct method. In: Official methods and recommended practices of the American Oil Chemists’ Society. American Oil Chemists Society, Champaign, IL
Day L, Seymour RB, Pitts KF, Konczak I, Lundin L (2009) Incorporation of functional ingredients into foods. Trends Food Sci Technol 20:388–395
de Man JM (1992) Chemical and physical properties of fatty acids. In: Chow CK (ed) Fatty Acids in Foods and their Health Implications. Marcel Dekker, New York, pp 17–46
EFSA Panel on Biological Hazards (BIOHAZ) (2010) Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J 8:1874
Gerber M (2012) Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr 107:S228–S239
Golmakani M-T, Moosavi-Nasab M, Keramat M, Mohammadi M-A (2018) Arthrospira platensis extract as a natural antioxidant for improving oxidative stability of common kilka (Clupeonella cultriventris caspia) oil. Turk J Fish Aquat Sci 18:1315–1323
Ichihara KI, Shibahara A, Yamamoto K, Nakayama T (1996) An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 31:535–539
IUPAC (1987) Method Number 2.504. Determination of the p-anisidine value (p-AV). In: StandardMethods for the Analysis of Oils, Fats and Derivatives. Blackwell, Boston
Jamnik P, Mahnič N, Mrak A, Pogačnik L, Jeršek B, Niccolai A, Masten Rutar J, Ogrinc N, Dušak L, Ferjančič B, Korošec M, Cerar A, Lazar B, Lovše U, Pungert T, Fabjan P, Poklar Ulrih N (2022) Fermented biomass of Arthrospira platensis as a potential food ingredient. Antioxidants 11:216
Kahraman G, Özdemir KS (2021) Effects of black elderberry and Spirulina extracts on the chemical stability of cold pressed flaxseed oil during accelerated storage. J Food Meas Charact 15:4838–4847
Kozłowska M, Gruczyńska E (2018) Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem Pap 72:2607–2615
Kuley E, Uslu L, Durmus M, Sakarya Y, Özyurt G (2023) Enhancement of Spirulina platensis bioactivity by probiotic fermentation and encapsulation by spray-drying. Int J Food Sci Technol 58:6015–6024
Liu J-G, Hou C-W, Lee S-Y, Chuang Y, Lin C-C (2011) Antioxidant effects and UVB protective activity of Spirulina (Arthrospira platensis) products fermented with lactic acid bacteria. Process Biochem 46:1405–1410
Mendiola JA, Jaime L, Santoyo S, Reglero G, Cifuentes A, Ibañez E, Señoráns FJ (2007) Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem 102:1357–1367
Morsy MK, Morsy OM, Elbarbary HA, Saad MA (2019) Enhancing of oxidative stability and quality attributes of olive oil using spirulina (Arthrospira platensis) nanoparticles. LWT 101:444–455
Niccolai A, Bažec K, Rodolfi L, Biondi N, Zlatić E, Jamnik P, Tredici MR (2020) Lactic acid fermentation of Arthrospira platensis (Spirulina) in a vegetal soybean drink for developing new functional lactose-free beverages. Front Microbiol 11:560684
Özogul İ, Kuley E, Durmus M, Özogul Y, Polat A (2021) The effects of microalgae (Spirulina platensis and Chlorella vulgaris) extracts on the quality of vacuum packaged sardine during chilled storage. J Food Meas Charact 15:1327–1340
Özyurt G, Sakarya Y, Durmuş M (2022) Chemical and physical characterization of spray dried fish oil with different combination ratios of wall component. J Food Process Preserv 46:e17223
Özyurt G, Uslu L, Durmuş M, Sakaraya Y, Uzlasir T, Küley E (2023) Chemical and physical characterization of microencapsulated Spirulina fermented with Lactobacillus plantarum. Algal Res 73:103149
Pérez-Alva A, MacIntosh AJ, Baigts-Allende DK, Garcia-Torres R, Ramirez-Rodriguez MM (2022) Fermentation of algae to enhance their bioactive activity: A review. Algal Res 64:102684
Romay CH, Gonzalez R, Ledon N, Remirez D, Rimbau V (2003) C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 4:207–216
Rosas VT, Monserrat JM, Bessonart M, Magnone L, Romano LA, Tesser MB (2019) Comparison of β-carotene and Spirulina (Arthrospira platensis) in mullet (Mugil liza) diets and effects on antioxidant performance and fillet colouration. J Appl Phycol 31:2391–2399
Shabbir MA, Mehak F, Khan MR, Ahmed W, Nawaz MF, Hassoun A, Bhat ZF, Aadil RM (2023) Unraveling the role of natural functional oils in modulating osteoarthritis related complications. Crit Rev Food Sci Nutr 10:1–21
Shahidi F, Zhong Y (2010) Lipid oxidation and improving the oxidative stability. Chem Soc Rev 39:4067–4079
Takei M, Kuda T, Eda M, Shikano A, Takahashi H, Kimura B (2017) Antioxidant and fermentation properties of aqueous solutions of dried algal products from the Boso Peninsula, Japan. Food Biosci 19:85–91
Takyar MBT, Khajavi SH, Safari R (2019) Evaluation of antioxidant properties of Chlorella vulgaris and Spirulina platensis and their application in order to extend the shelf life of rainbow trout (Oncorhynchus mykiss) fillets during refrigerated storage. LWT 100:244–249
Yeşilsu AF, Özyurt G (2019) Oxidative stability of microencapsulated fish oil with rosemary, thyme and laurel extracts: A kinetic assessment. J Food Eng 240:171–182
Wu HL, Wang GH, Xiang WZ, Li T, He H (2016) Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int J Food Prop 19:2349–2362
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The Scientific Research Projects of Cukurova University (FBA2024/15886) in Turkey provided financial support for this work.
Author information
Authors and Affiliations
Contributions
GÖ: Project administration, Conceptualization, Writing–original draft, editing. MD: Investigation, Formal analysis. YS: Methodology, Formal analysis. LU: Formal analysis, Methodology. EK: Investigation, Writing-review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Özyurt, G., Durmuş, M., Sakarya, Y. et al. Improved oxidative stability of functional oils with Spirulina enhanced by probiotic fermentation and encapsulation. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03209-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03209-x