Abstract
Wastewater from the potato processing industry called protamylasse is rich in proteins and carbohydrates that potentially can be valorized through cultivation of microalgae by mixotrophic metabolism. However, the complex organic compounds are a challenge, as algae grow best on simple compounds such as volatile fatty acids (VFA). This study demonstrates a new two-stage system. First, VFA production was achieved by testing mesophilic and thermophilic anaerobic acidification (AA) at a short hydraulic retention time (HRT; 3.3 and 5 days) resulting in the release of ammonium and phosphate. HRT of 5 days and thermophilic conditions was optimal considering the high acetate yield of 0.23 g and 22 ml CH4 per g volatile solids (VS). Then, Chlorella sorokiniana was chosen based on the obtained growth rate, and better adaption in ammonium-rich AA effluent after screening several tested microalgae (Chlorella sorokiniana, Chlorella vulgaris, Scenedesmus obliquus, and Haematococcus pluvialis). It was cultivated for valorization of nutrients and organics and successfully upscaled to 25 L photobioreactor (PBR) scale under both batch and continuous operation with high dosage of 25% (8.2 g L−1 of VS) of AA effluent at an HRT of 5 days in the PBR. Chlorella sorokiniana removed more than 99% of the chemical oxygen demand (COD) and the VFA during continuous flow PBR operation. This approach contributed to the final removal efficiency of 71%, 91%, and 78% for phosphorus, nitrate, and ammonia, respectively, and production of microalgae biomass with more than 73% protein. Thus, a promising process for simultaneous treatment of high strength wastewater for microalgal protein production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Declining fossil fuel reserves and the need to prevent climate change have led to major efforts to develop feedstocks for energy, chemicals, and materials derived from renewable resources (Chaudry et al. 2015). The initial effort focused on land-based crops, which are not sustainable as they compete with edible crops for arable land, freshwater, and fuel for the implemented agricultural practices. Large-scale microalgae cultivation has therefore emerged as a promising approach for sustainable and carbon–neutral production of biofuels and high valued products (Nwoba et al. 2019). Microalgae show great potential for the production of biofuels (Chisti 2008) and food supplements such as protein and antioxidants (Batista et al. 2017). The cultivation approach can be made even more sustainable by the use of residual resources (e.g., industrial wastewaters) as an alternative cultivation medium (Maurya et al. 2022).
The food industry generates various high-strength organic sidestreams (wastewaters) with great potential for fermentation purposes (Hamza et al. 2018). For potato starch production for example, 3.5 t of wastewater are generated per tonne of starch produced mentioned as protamylasse. This protamylasse is produced by evaporation of water from potato juice as described by Fang et al. (2011). Potato juice is a residue formed by coagulation and separation of the potato protein content. The protamylasse contains large amounts of degraded protein and peptides with high fractions of the amino acids aspartic acid and glutamic acid (Elbahloul et al. 2005). Nowadays this wastewater is used as fertilizer on agricultural soils. However, alternative treatment methods can provide more value to this sidestream and thereby improve the overall economy of the production process.
Microalgae can be used for wastewater treatment to remove chemical oxygen demand (COD), ammonium, and phosphate by mixotrophic growth, along with production of various valuable compounds such as protein and pigments (Pan et al. 2021). The simultaneous bioremediation of wastewater prior to discharge to the environment is thereby an extra advantage of wastewater treatment by microalgae (Renuka et al. 2015). Microalgae showing great potential for resource recovery include Chlorella sorokiniana (Lizzul et al. 2014; Rude et al. 2022), Chlorella vulgaris (Ren et al. 2022; Rude et al. 2022), Scenedesmus obliquus (Rude et al. 2022), and Haematococcus pluvialis (Pan et al. 2021). Chlorella sorokiniana is highly valued in the microalgal industry for its adaptability, robustness, and fast growth rate. Its biomass is considered valuable as a food additive for humans due to its high protein content, an abundance of vitamins, pigments, antioxidants, and other bioactive compounds of high value (Chia et al. 2015; Shim et al. 2020). Chlorella vulgaris is mostly suggested for biodiesel production (Rude et al. 2022) and H. pluvialis is known for astaxanthin production but it is sensitive to cultivation conditions and often challenging to grow in wastewater. Scenedesmus obliquus can produce protein for food supplements (de Silva et al. 2021).
Unfortunately, many algae cannot grow well on untreated protamylasse due to its high content of complex carbon sources and organic-bound nutrients. Therefore, pretreatment is needed to decompose the organic substances into simple compounds and inorganic nutrients (Maurya et al. 2022), which can be achieved in anaerobic digestion (AD). The AD process consists of four steps including hydrolysis, acidogenesis, acetogenesis, and finally methanogenesis. First, depolymerization and solubilization of polysaccharides and proteins is performed by hydrolytic bacteria (Li et al. 2011). Then, acidogenic bacteria decompose the hydrolysis products into volatile fatty acids (VFA) ranging from acetic acid to hexanoic acid. Afterward, acetogenic bacteria transform the long-chain VFA into acetic acid. Anaerobic acidification (AA) is thus achieved by omitting the methanogenesis step at low HRT with VFAs as main product (Gautam et al. 2021). VFA are of interest for microalgal cultivation because they can be utilized during mixotrophic growth (Pan et al. 2021).
The present study investigates a new strategy to valorize the protamylasse by using it as a carbon and nutrient source for microalgal cultivation. AA was tested as a pretreatment method in order to convert the complex organic matter into VFA, which could subsequently be used for the cultivation of microalgae. Cultures of C. sorokiniana, C. vulgaris, H. pluvialis, and S. obliqus were screened against different AA effluent dilutions to select a robust strain. After screening, the selected microalga was assessed for its ability to remove nutrients and COD from the protamylasse during the production of algal biomass in 1-L flasks. Afterward, the cultivation was upscaled in a 25 L photobioreactor (PBR) under the best cultivation conditions and the algal composition was assessed for carbohydrate and protein content.
Materials & methods
Anaerobic acidification reactor experiments
Inocula and feedstock
The protamylasse was collected from the potato starch production plant at KMC in Brande, Denmark, in September 2021 and stored at -20 °C before use. The protamylasse was produced by evaporation of water from potato juice achieved at the factory under vacuum at 80 °C used for protein coagulation. This process resulted in 100 g protamylasse, 900 g water and coagulated protein residue per kg potato juice. The protamylasse is considered in this work. The anaerobic acidification feedstock was prepared by mixing water and protamylasse in a ratio of 4.565 g water g−1 to achieve a volatile solids (VS) concentration of 40 g L−1. The mesophilic inoculum was collected at Hashøj Biogas, DK, while the thermophilic inoculum was collected at Lemvig Biogas. The two inocula were degassed (i.e. incubated at process temperature until biogas production ceased) before the reactor operation.
Anaerobic acidification reactors description and operation
The AA process was conducted in a continuously stirred-tank reactor (CSTR) with a total volume of 2.0 L and a 1.8 L working volume for 125 days as described by Fotidis et al. (2014). The reactor and the feeding bottle were mixed with magnetic stirring and a peristaltic pump (Watson Marlow-620 series) was used to feed the reactor. A flask for harvesting the digestate and a water replacement gas meter was connected to the reactor. The gas was not pretreated before the analysis, thus the mixture of CH4, CO2, and H2 was measured in collected gas samples. The parameter set was the same for mesophilic and thermophilic reactors except for temperatures of 37 °C and 55 °C, respectively. The HRT was 5 days during the first 40 days. Thereafter, it was reduced to 3.3 days for 40 days and finally (last 45 days) it was changed to the initial setting of 5 days. M3.3 and M5.0 refer to mesophilic conditions with 3.3- and 5.0 days HRT, respectively, while T3.3 and T5.0 refer to the same HRTs at thermophilic conditions. During the first 4 days of the experiment, an adaptation period was applied as follows to keep a 5 day HRT. On day 1, 900 mL inoculum + 180 mL feed was added and on days 2, 3, and 4, 216 mL, 259- and 311-mL feed were added. Thereafter, 360 mL and 540 mL feed per day were applied at HRT at 5 and 3.3 days, respectively, using 180 mL pulses. The gas composition was measured in the outlet and liquid samples were taken from the reactor for pH and VFA analysis.
Microalgae cultivation
Inoculum and synthetic media
The algal strains Chlorella vulgaris CCAP 211/11b, Chlorella sorokiniana CCAP 211/8 K, and Scenedesmus obliquus SAG 2327, were cultivated in BG-11 medium and Haematococcus pluvialis SAG 192.80 was cultivated in BBM (see Table S1; Ilavarasi et al. 2011). Inocula were cultivated in a 250 mL flask with 100 mL of media on a shaker at 150 rpm and a temperature of 25 °C. Day and night cycles of 12 h were applied with an illumination of 100 µmol photons m−2 s−1. In all experiments, the inoculum was prepared and retrieved from the exponential growth stage.
Anaerobic acidification effluent as a media for microalgae cultivation
The effluents from the AA CSTRs were firstly centrifuged at 4,000 rpm for 10 min to remove large undissolved particles in order to avoid blockage of tubes, Thereafter, the supernatants were autoclaved at 121 ℃ for 20 min to ensure no microbes were active. As a preliminary study, the T5.0 effluent was chosen as a cultivation medium for microalgae, due to its high acetic acid concentration (Table 1). This AA effluent was tested as a growth medium for the four microalgae strains. The AA effluent concentration utilized as a growth medium was optimized used at 100, 10, 5, 3.3, and 2.5% final concentration. For the screening test, the inoculation was achieved by adding 0.2 mL of inoculum into 24 well microtiter plates with the final 2 mL working volume as batch cultures (Van Wagenen et al. 2014). The 1 L Erlenmeyer flasks experiments (500 mL working volume) were also accomplished by inoculation at a ratio of 1:10 (inoculum: working volume). The flasks were filled to the total volume (500 mL) by applying the four AA effluents, separately. Subsequently, all the flasks were placed on a shaker operating at 100 rpm and kept at room temperature (25℃), with a light intensity of 100 µmol of photons m−2 s−1 and light/dark ratio of 12 h/12 h with white light, (LED, Ledvance, Denmark). To maintain sterility, ventilating-sterilized-membranes were employed to cover all flasks. All the batch experiments were done in triplicate.
Photobioreactor experiments
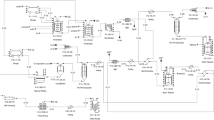
A 25 L airlift PBR with 20 L working volume made of Plexiglas (Fig. 1) was operated initially in batch and subsequently in continuous flow mode. The PBR was equipped with temperature, pH, dissolved oxygen (DO) and total solid (TS) probes. Outlet for air was fitted on the top of the PBR, while an outlet sample valve was fitted on the bottom. Air inlet was fitted in the bottom of the PBR through a plate diffuser in 0.025 vvm. LED lights with an intensity of 80 µmol of photons m−2 s−1 were fitted around the PBR’s outer wall (360 Round neon led light strip 220 V, 2835 Tube flexible rope light (Shenzen Oumuka Technology Co. Limited, China). A CO2 inlet was used to control the pH value at 8 ± 0.2. An antifoam syringe was applied on the top of the PBR to reduce foaming.
Initially, a 5 L flask was used as an intermediate upscaling stage to achieve sufficient cell density for cultivation. After five days, at the beginning of the exponential phase C. sorokiniana was inoculated to the PBR with (1:10) inoculum: media ratio and filled to 20 L (working volume) with BG-11 medium. The batch experiment lasted 5 days and samples were retrieved every day. While the algae culture was in the exponential phase, 1 L of the culture was harvested and replaced by 1 L protamylasse resulting in 5% protamylasse in the PBR.
The continuous operation was started when the maximum growth rate was observed corresponding to a threshold value of 0.3 day−1. However, in order to avoid cell wash out in the PBR the dilution rate was only 0.25 day−1 (5 days HRT) in the continuous flow experiment which lasted for 13 days (> 2 HRTs). The first HRT cycle was fed with 3.3 L synthetic medium and 0.7 L of protamylasse per day and the second cycle upscaled the protamylasse to 1 L and synthetic medium down to 3L per day with a PBR working volume of 20 L. The molar nitrogen to phosphorus (N/P) ratio of the feed was varied during the continuous cultivation. During the first 5 days the N/P molar ratio was kept at 19.9 ± 1.2, based on Wágner et al. (2021). Then, the N/P ratio was increased to 23.33 ± 0.1 until the end of the operation. Synthetic medium and acidified protamylasse were fed by two peristaltic pumps, using 20.8 mL wastewater and 62.5 mL synthetic medium per pulse with 48 pulses per day.
Analytical methods
General analysis
Standard methods were used to determine total solids (TS), VS, and COD (Aniyikaiye et al. 2019). The gas composition was analyzed by a gas chromatograph (Trace 1310 GC-TCD, Thermo Fisher, USA) with He as carrier gas, which is equipped with Thermo (P/N 26004–6030) column (30 m length, 0.32 mm I.D. and film thickness 10.0 μm). CO2 and CH4 were measured on a Q-plot column while H2 was measured on an HP-Molesieve column. COD, PO43+ and NO3− were measured using the chemical kits LCK014, LCK350 and LCK339, respectively (Hach Lange ApS, Denmark). NH4+ was measured by distillation with HCl (Vapodest 450 (GER12-0545), LabDanmark, Denmark) while Total Kjeldahl Nitrogen (TKN) was measured with the Kjeldatherm digestion system (GER12-0729, LabDanmark, Denmark). pH was measured using a FiveEasy Plus pH meter (Mettler Toledo, Denmark). VFA concentrations were measured using Agilent 7890A gas chromatograph (Agilent Technologies, USA) equipped with a flame ionization detector (FID) and SGE capillary column (30 m length, 0.53 mm I.D. and film thickness 1.00 µm) with He as carrier gas.
Growth analysis of C. sorokiniana
The percentage of nutrient removal in the algal cultures was determined using the following equation:
Algal growth was assessed in terms of optical density (OD), dried cell weight (DCW), and the in vivo fluorescence (IVF). The growth was measured at the highest wavelength, 680 nm, achieved by spectrum scanning and suggested in the literature (Rude et al. 2022). IVF using an excitation wavelength at 440 nm and an emission wavelength at 690 nm was measured in a Synergy H1M microplate reader (Agilent, Denmark). Dissolved oxygen and pH were measured using the WTW sensors FDO925 and Sentix 980, respectively, connected to a WTW Multi 3480 receiver. Biomass productivity of microalgae was measured by daily filtering 5 mL from the PBR through a pre-weighed 2.5-μm GF/C filter (Whatman). Before filtration, the GF/C filters were washed in deionized water and dried at 75 °C for 24 h, and the dry weights of the filters were determined. Then, the filters were dried in an oven at 105 °C and the biomass productivity was obtained by Eq. (2) (Mohamadnia et al. 2022a).
where W0 and Ws are the biomass concentration (g L−1) after inoculation and at any specific time during the continuous cultivation (ttot = total measurement days), respectively.
Biochemical composition of C. sorokiniana
At the end of cultivation, microalgal cultures were separated by centrifugation (10,000 rpm, 5 min, 4 ºC) and then freeze-dried to obtain algal powder. The powder was then employed to analyze the contents of proteins, lipids, and carbohydrates in algal cells. Protein was measured according to Naseri et al. (2020) using DUMAS nitrogen/protein analyzer a fully automated rapid MAX-N exceed (Elementar Analysensysteme GmbH, Germany). Finally, the protein content was calculated by multiplying the nitrogen content by a conversion factor of 6.25 (Wágner et al. 2021). Total carbohydrates and total lipids were determined according to phenol–sulfuric acid and sulpho-phospho-vanillin (SPV) colorimetric methods, respectively (Mohamadnia et al. 2021).
Calculations
The protein concentration in the AA effluent was calculated based on the TKN, the ammonium concentration, and the conversion factor (0.1459 g N g protein−1; Table S2). The TKN was assumed to be constant in the AA process.
The algal yield was calculated based on the TS content in the feed and the input flow rate, considering the mass of algal biomass produced.
Statistics
All analysis including TS/VS, VFA, pH, and gas composition were measured in duplicate. Significance was analyzed for the parameters in Table 1 as 2-way ANOVA with meso/thermo as parameter 1 and HRT as parameter 2. The determined results are effect of parameter 1, parameter 2 and their combined effect.
Results
Anaerobic acidification experiments
Protamylasse composition
The overall protamylasse composition including the fractions based on the TS content is shown in Table S3. It contained high VS (250 ± 2 g L−1), N and phosphate concentrations. After dilution, the resulting AA feed contained 40 g L−1 VS and 52 g L−1 COD. The nutrients included 3.79 g L−1 TKN of which 0.73 g L−1 was ammonia and 0.81 g L−1 phosphorus of which 0.33 g L−1 was on inorganic form (Table 1). The measured organic compounds were 21.0 g L−1 protein and 6.3 g L−1 starch contributing to 68% of the VS content.
Carbon conversion into VFAs and biogas
The VFA production rate per L reactor volume is shown in Fig. 2 while the achieved VFA concentrations are shown in Table 1. The VFA average concentrations are based on steady state considering day 20–38 and day 83–125 for HRT 5.0 and day 41–80 for HRT 3.3 including 20 data points per HRT value. The total VFA production rate increased during the first 10 days (2 HRT) due to adaptation to the protamylasse reaching 2.8 g L−1 day−1 (Fig. 2a). Thereafter the rate increased slightly to 3.4 g L−1 day−1 after 40 days and it was similar at mesophilic and thermophilic conditions. After 40 days the HRT decreased from 5.0 to 3.3. days−1, which resulted in big oscillations with rates between 3.5 and 5.5 g L−1 day−1. This shows that the process was less stable presumably due to more stressful conditions for the microbial community. After 80 days the HRT was changed back to 5 g L−1 day−1, which reduced the oscillations with rates between 2.9 and 3.2 g L−1 day−1. Based on average values in Table 1, the total VFA concentration was highest at 5 days HRT and mesophilic conditions (18.2 g L−1) presumably due to a longer time for the acidification process. The effect of HRT was significant (p > 99.9%) while there was no significant effect of temperature as shown with ANOVA 2-way analysis.
The VFA production rates in the mesophilic (M) and the thermophilic (T) reactor vs time (a and b) are shown as mean ± standard deviation (n = 3). The volumetric gas production rates are shown in (c). The HRT 5 set was for days 0 – 40 and again for days 81 – 125 while the HRT 3.3 set was for days 41 – 80
Among the individual VFAs, acetic acid was produced at the highest rate of 2 g L−1 day−1 at thermophilic conditions (Fig. 2a). Higher oscillations were achieved at HRT of 3.3 days in the range 2 to 2.8 g L−1 day−1. At mesophilic conditions, a lower acetic acid production was achieved of 1.6 g L−1 day−1 using HRT of 5 days. The achieved acidic acid concentration was thereby positively affected (1 g L−1 higher) at thermophilic conditions and at the long retention time (1 g L−1 higher) with a maximum for T5.0 at 9.2 g L−1 (Table 1). This concentration corresponds to a yield of 0.23 g per g VS. Both the effects of HRT and temperature (M/T) were significant (p > 99.9%).
The propionic acid concentration was highest at mesophilic condition (4.08 g L−1) with a significant effect of HRT and temperature (M/T). The butyric acid concentration was significantly higher at HRT of 5 days (4.90 g L−1, Table 1). The valeric acid and the hexanoic concentration were in general low (0.3 – 0.7 g L−1). The corresponding production rates were slightly higher at HRT on 3.3 days (0.08 – 0.25 g L−1 day−1) than at 5.0 days (0.04 – 0.21 g L−1 day−1) and highest at the mesophilic conditions (Fig. 2b). The general result was that the acetic acid fraction of the VFA was highest at thermophilic conditions (52 – 54%) presumable due to more effective acetogenesis at these conditions (Table 1).
The CH4 production rates are shown in Fig. 2c while the yields are shown in Table 1. The CH4 production rates are shown in Fig. 2c while the yields are shown as average concentrations in Table 1. The average concentrations for both CH4 and CO2 are based on steady state considering day 15–24 and day 91–107 for HRT 5.0 and day 91–107 for HRT 3.3 including 25 data points per HRT value. At mesophilic conditions, the CH4 yield was 10 mL g−1 VS while it was significantly higher (22 mL g−1 VS) at thermophilic conditions (at HRT of 3.3 days). The methane yield was slightly but not significantly lower at HRT of 5 days. The CH4 production rate was fluctuating but highest during the initial 15 days followed by a stable level of 250 mL L−1 day−1 at thermophilic and 100 mL L−1 day−1 at mesophilic conditions. The CO2 production rate was slightly higher at thermophilic conditions (600–900 mL L−1 day−1) than at mesophilic conditions. The CO2 yield was thus significantly higher at thermophilic (55 mL g−1 VS) than at mesophilic conditions (45 mL g−1 VS) while there was no effect of HRT. However, the yield-based methanogenesis was similar and very low compared to typical AD processes conducted at HRT above 15 days.
The COD concentration decreased from 52 g L−1 to 45 and 48 g L−1 at mesophilic conditions and thermophilic conditions, respectively while the VS decreased from 40 to 34 and 33 L−1, respectively. The decrease of COD was expected due to the production of CH4 and H2 in the AA process. The decrease in starch and protein concentration was 5 and 8 g−1 VS, respectively and partly contributing to the VFA production (Table 1).
Nutrient release by acetogenisis
The nutrient concentrations are shown in Table 1. The NO3− concentration decreased from 78 mg N L−1 to 44 and 51 mg N L−1 during the AA process under mesophilic and thermophilic conditions, respectively. At the same conditions the NH4+ concentration increased from 730 mg N L−1 to 2000 and 1920 mg N L−1, respectively, as a result of amino acid degradation. ANOVA analysis of IVF showed no significant effect of temperature and HRT but there was a combined effect (p > 95%). The highest IVF was thus achieved with M5.0 and T3.3 (6409–6437). The NH4+ concentration correlated with IVF, confirmed by regression analysis (R2 = 0.997). The T5.0 effluent was selected for the continued study because it was richest on acetate (9.17 g L−1). The effect of both HRT and temperature were significant (p > 99.9%) as found with 15 data points at each HRT value. Day 20–38 and 94–125 were used for HRT 5.0 while day 48–83 were used for HRT 3.3.
The ammonium production had greatly neutralized the produced VFAs, thereby preventing pH values to decrease to below 6.4 in the AA process (Fig. S1b). The inorganic phosphate concentration increased due to its release by degradation of organic compounds such as nucleic acids and mostly at 5 days HRT to the range of 500 to 580 mg P L−1.
Microalgal cultivation
Screening in 24 well microtiter plates
The four effluents were compared at a dosage of 10% for cultivation of C. sorokiniana and the fluorescence (IVF) and OD were shown in Fig. 3a. The graph shows similar rates of increase for all four AA effluents during the first 4 days. Thereafter the IVF stabilized at 6000 and the OD at 1.1 – 1.2. ANOVA analysis of IVF showed no significant effect of temperature and HRT but there was a combined effect (p > 95%). The highest IVF was thus achieved with M5.0 and T3.3 (6409–6437). The NH4+ concentration correlated with IVF, confirmed by regression analysis (R2 = 0.997). The T5.0 effluent was selected for the continued study because it was richest on acetate (9.17 g L−1). T5.0 effluent was diluted and used for the cultivation of C. sorokiniana in 24 microtiter plates at 0, 10, 20, 30, 40 times dilution of the protamylasse for 5 days corresponding to dosage of 100, 10, 5, 3.3, and 2.5%, respectively (Fig. 3b). The achieved IVF increased along with OD in all the cases is shown in Fig. 3a. Based on this preliminary result, screening between the four microalgae strains and different dilution dosage of T5.0 effluent was accomplished (Fig. S2), which shows that the Chlorella spp. performed better than S. obliquus and H. pluvialis. It can be seen that C. sorokiniana reached the highest OD (1.4 ± 0.11) with no apparent lag phase in less time of cultivation (4 days) rather than C. vulgaris (6–7 days). Thus, C. sorokiniana was chosen as a representative of Chlorella as explained by its robustness in high organic content wastewater (Rasouli et al. 2018; Asadi et al. 2019).
Cultivation in 1-L flasks
The growth of C. sorokiniana in terms of OD and IVF in the T5.0 effluent in 1-L flasks is shown in Fig. 4a. During the 5 day cultivation period, the COD concentration was reduced from 4300 mg L−1 to 0 (Fig. 4b). Besides, the microalgae consumed phosphate which resulted in gradual decline vs. time, from 145 mg L−1 to below 50 mg L−1 after 4 days (Fig. 4c). The main N source in the AA effluent was ammonia, which after four days was reduced to 3 mg NH4+-N L−1, while the NO3− concentration remained constant until all the ammonium was consumed.
Scale up the cultivation to the 25-L photobioreactor
The IVF and OD in 25 L batch cultivation of C. sorokiniana are shown in Fig. 5a and the result thereby confirmed a high fluorescence at the 25 L scale as on the 2 mL and 1 L scales. The nutrient concentrations depicted in Fig. 5b indicate that ammonium was initially consumed followed by nitrate removal. Furthermore, to be on the safe side of the experiment and avoid shocking the microalgae cells with high organic loading at the beginning, the batch experiment was initiated with the 5% dilution dosage of T5.0 effluent. Thus, acetic acid was removed on the first day, followed by degradation of the other VFAs (Fig. 5c). Almost all the COD was removed at day 3 resulting in a steady increase in IVF due to the algal growth (Fig. 5c).
Cultivation of Chlorella sorokiniana in the 25 L PBR. The plots a) (OD and IVF), b) (nutrients), and c) (COD and VFA) in the left column represent the batch experiment using 5% T5.0. Similarly, d), e), and f) represent the continuous experiment using 25% T5.0. Data are shown as mean ± SD (n = 3). The COD load in the continuous experiment was 3110 mg L−1 until day 4 and thereafter increased up to 4670 mg L−1
Figure 5d presents OD, dry cell weight (DCW), and the IVF of microalgae in the T5.0 effluent in continuous flow mode. The biomass concentration of C. sorokiniana increased to 1.025 ± 0.16 g L−1 and 1.51 ± 0.028 g L−1 on day 5 and day 10, respectively, which corresponded with the end day of the first and the second HRT (Fig. 5d). Moreover, the biomass production rates were 0.25 and 0.11 g L−1 day−1, respectively, throughout the two HRTs applied for the continuous cultivation. The maximum biomass obtained on day 8 which corresponded to 1.925 ± 0.07 g L−1 (Table S4). The IVF was 5000 during the first 4 days at the T5.0 dosage of 17.5% and at the 25% dosage it decreased to 3000. However, the OD continued to increase and reached a plateau (Fig. 5d). The 25% dosage corresponds to a feeding of 8.2 g L−1 of VS (Table 1). Nutrient concentrations in continuous operation are shown in Fig. 5e. The COD and VFA profile during the continued cultivation are shown in Fig. 5f. For reference, in the initial 4 days, 3000 mg L−1 of COD was added followed by 4700 mg L−1 in the subsequent 7 days. This resulted in increasing COD concentrations until day 9 of 2300 mg L−1 followed by a rapid decline to 200 mg L−1 at day 11. Total VFA removal was achieved until day 6 followed by increasing concentration at day 7 – 8 and finally a decrease to 0 at day 11 (Fig. 5f).
Finally, C. sorokiniana after 11-days of continuous cultivation was collected and analyzed (Fig. 6). The proteins, lipids, and carbohydrates contents were 73.6%, 4.7%, and 18.8%, respectively.
Discussion
The anaerobic acidification process
The anaerobic acidification (AA) process had the goal to decompose the complex polymeric organic molecules from waste materials (carbohydrates, lipids, and proteins) to VFAs (Lukitawesa et al. 2020). In this study we used anaerobic processes at low HRT to convert the polymeric compounds to VFA. In general, the main contributors for VFA production in anaerobic fermentation include diverse hydrolytic and acidogenic bacteria (He et al. 2019), which are responsible for complex organic degradation and VFA bioconversion. In the anaerobic digestion process these VFA are typically converted to biogas by microbial conversion conducted by acetogenic and methanogenic microbes. However, methanogenesis is usually a much slower process, requiring much longer HRT (> 15–20 days).
Therefore, it was assumed by keeping the HRT low, the slow-growing methanogens are washed out, which led to high VFA levels in the effluent. However, it was contradicting that the CH4 production rate in both mesophilic and thermophilic conditions was higher at HRT of 3.3 than at 5 days which can be attributed to higher feed flow rate. There was a significant difference between thermophilic and mesophilic conditions in VS-based methane yield (21.55 and 9.5 mL g−1 VS, respectively) while similar yields were obtained in different HRTs (Table 1). The yields were thereby much lower than the CH4 potential for protamylasse reported by Fang et al. (2011) of 492 mL based on 100% conversion of the COD content. These VFAs could subsequently be used for heterotrophic/mixotrophic cultivation of microalgae. Ammonia and phosphates released by decomposition of organics can play an important role during the subsequent algal cultivation, ensuring right C/N/P ratios for production of microalgal biomass (Procházka et al. 2012). An extra benefit is the buffering system in the anaerobic digester achieved from the interaction among 3 buffers: VFA (acetic acid with a dissociation constant, pKa, of 4.8), bicarbonate (CO2/HCO3 − with a pKa of 6.4), and ammonia (pKa of 9.25) (Georgacakis et al. 1982). The produced VFA are thus neutralized due to the associated release of ammonia in the process.
Nitrate removal in the AA process was not complete (Table 1). It could be expected due to the anaerobic conditions. However, the study by de Sousa et al (2008) confirms that complete denitrification was only achieved to 90% extend. This indicates that the inoculum was weak on denitrifying bacteria.
Microalgal cultivation
Growth and biomass productivity of C. sorokiniana. Algal biomass is considered a highly promising biomass due to its diverse applications as biofuels, biofertilizers, and useful food ingredients. The growth pattern of C. sorokiniana exhibited typical characteristics of microbial growth. The growth pattern of C. sorokiniana in both small batch cultivations (2 mL and 1 L) exhibited an initial brief adaptation period followed by an exponential phase characterized by mixotrophy as witnessed by the removal of COD, and finally an autotrophic phase. In contrast, during continuous flow operation, organic compounds were supplied during the entire cultivation. Hence the mixotrophic condition was established throughout the whole experiment.
Chlorella can be produced mixotrophically in a closed system with improved contamination control from other microbes and optimal culture conditions to increase biomass yield (Ende and Noke 2019). Although the systems used in this study were previously sterilized, bacteria were observed alongside the microalgae strain. Even in these circumstances, C. sorokiniana grew without interruption for days even while the aerobic microbe count, observed by microscopy, decreased. Consequently, it may be assumed that in the studied conditions, microalgal growth may be able to manage these bacteria. Also, prior studies have shown that Chlorella strains were part of a consortium competing with other microalgae and exhibited biocidal activity against bacteria (Lois-Milevicich et al. 2020). Nevertheless, the high COD, nitrogen, and phosphorous content of protamylasse may lead to adverse effects such as reduction of DO content in water and result in limited mixotrophy conditions. Indeed, the dark brown color of the protamylasse can prevent light penetration resulting in almost heterotrophic condition inside the PBR, which means the autotrophic metabolism of microalgae has not had enough contribution to supply the oxygen needed for mixotrophic growth (Cai et al. 2013). Furthermore, the biofilm formation of C. sorokiniana at the top of the air diffuser (Fig. S3) limited the oxygen availability in the PBR and diminished the growth resulting in low dry cell weight at the steady state condition. Specifically, in cases where the substrate exhibits a high degree of coloration, adequate mixing is crucial in order to ensure that the microalgae can receive sufficient light. Otherwise, the light will only be available at the surface of the substrate (Marcilhac et al. 2014). The coloration of a medium can impede the autotrophic growth of microalgae by limiting light penetration. To promote light penetration, dilution is required which can reduce nutrient content that could hinder microalgae growth. In addition to the color of the media, high concentrations of microalgae may also reduce light penetration. The achievement of the current study was in accordance with the other study in terms of the superiority of Chlorella over Scenedesmus under conditions of limited light availability (Marcilhac et al. 2014). Furthetmore, the ammonia content of protamylasse after dilution was much higher than the inhibitory level (143 mg L−1), which could be a source of toxicity for microalgal growth (He et al. 2013). This was also verified by Rude et al. (2022) who found C. sorokiniana may be preferred for biomass cultivation on ammonia-rich ultrafiltered anaerobically digested food waste over C. vulgaris and S. obliquus, which both demonstrated longer apparent growth periods and lower biomass productivity overall. On the other hand, they achieved 0.44 ± 0.03 day−1 growth rates for C. sorokiniana cultivated in 10 times dilution of ultrafiltered anaerobically digested food waste from the thermophilic digester in 250 mL flask which might be due to the lower amount of ammonia concentrations (lower toxicity) and transparent color rather than the current study (0.28 in a 1-L flask) (Rude et al. 2022).
Hence, optimizing the cultivation condition is a critical endeavor for each algal strain to achieve high biomass productivity in a short cultivation period.
Nutrients removal
The evaluation of nutrient consumption and effluent quality was conducted in both 1 L batch flasks and in a 25-L PBR (batch and continuous) cultivation of C. sorokiniana. The nutrient removal efficiency of microalgae is subject to several significant factors including the characteristics of wastewater, the light intensity and cycle of light exposure, the C/N/P ratio, the supply of carbon dioxide, and the mode of cultivation (Li et al. 2019). Microalgae usually prefer ammonium as a nitrogen source because redox reaction during assimilation is not required (Ramanna et al. 2014), thereby lowering the energy consumption. In the present study, all nutrients except nitrate were consumed at the end of the batch cultivations in both 1-L flasks and 25-L PBR. This is in accordance with findings from other studies about microalgae preference in presence both nitrate and ammonium availability. For instance, Cui et al. (2020) discovered that when C. sorokiniana was cultivated in diluted chicken farm flushing wastewater, the highest removal efficiencies of ammonium-nitrogen (NH4+-N), TN, TP, and chemical oxygen demand (COD) were 93.2%, 84.5%, 79.2%, and 95.8%, respectively. Removal efficiencies for NH4+-N, TN and TP ranging from 79 to 98% and for COD from 67 to 86% have similarly been reported during cultivations of C. sorokiniana using cockle wastewater and cooking cocoon wastewater. (Do et al. 2021; Xue et al. 2021; Yang et al. 2022). The study by Yang et al. (2022) was conducted using lower initial concentrations than protamylasse in the current study. Based on batch cultivation results, when upscaling to 25L continuous cultivation the N/P ratio in the feed was adjusted, so the microalgae did not suffer from nitrate accumulation inside PBR. Thereby, nitrate concentration in the effluent was almost constant at 31 ± 1.4 mg L−1. On the other hand, the optimized N/P ratio in the feed contributed to more protein production as a main bioactive compound derived from the C. sorokiniana biomass.
In contrast, the COD and ammonium concentration during the continuous cultivation in 25 L PBR increased until the middle of the second HRT. The combined effect of high dosage of the T5.0 effluent (up to 25%) in the feed and cell growth inside the PBR lead to a dark colour and shadow effect which nearly resulted in heterotrophic conditions. On the other hand, the microalgae in the heterotrophic process take up the organic carbon by aerobic respiration (Perez-Garcia et al. 2011; Yahampath Arachchige Don and Babel 2021). A drawback of the approach was biofilm formation at the top of the air diffuser (was not recognized during the cultivation) which resulted in a low oxygen availability. Although an increase in aeration rate would improve mass transfer, gas exchange, liquid agitation, it could cause shear stress to algae cells (Mohamadnia et al. 2022b) so the high aeration rate was not applied from the beginning of the continuous cultivation when the cell density were not high enough. On day 9, the daily aeration rate was increased up to 0.25 vvm (10 times greater) which was followed by increasing COD and ammonium removal. Afterwards, sudden reduction in both NH4+ and COD was observed which verified heterotrophic growth and aerobic respiration responsible for taking up the organic compounds by the microalgae. Similar observations have been reported in previous studies (Perez-Garcia et al. 2011; Yahampath Arachchige Don and Babel 2021).
Biochemical composition of microalgal biomass
Species of microalgae, particularly those within the genus Chlorella, often contain a variety of active components, including proteins, carbohydrates, lipids, and pigments. These compounds have value for a wide range of applications such as for feed, food, medical, pharmaceutical or fuel industries. It was observed that the chemical composition of C. sorokiniana varied in response to alterations in the nutrient profiles of wastewater, reactors and cultivation conditions. Generally, the microalgae cultivated under autotrophic and mixotrophic conditions, have higher protein contents than those achieved under heterotrophic (Lois-Milevicich et al. 2020). The intracellular nitrogen pool is dominated by protein, which can be affected by the availability of nitrogen in the surrounding medium, as noted by Zhao et al. (2009). Thus, in order to enhance the protein content of algae, it is advisable to maintain the N/P ratio between 20 and 30, provided that the medium contains adequate nitrogen concentrations for protein synthesis (Rasdi and Qin 2015; Do et al. 2021). The protein content in the current study (74%) is in agreement with the earlier research that reported a high protein level could be achievable with a N/P ratio in the range from 18 to 50 due to promotion of protein synthesis by elevated ambient N levels at these N/P ratios (Downing et al. 2005). However, it should be noted that high N/P ratios may lead to incomplete nitrogen removal, requiring further water treatment prior to discharge to the environment. Xue et al. (2021) also reported that when C. sorokiniana grown in a 300 mL bubble-column PBR feeding with cooking cocoon wastewater, the higher portion of the biochemical composition in microalgae biomass was proteins (41–49%).
The lipid content of the C. sorokiniana biomass was found to be significantly lower (4.7%) than that of standard lipid sources, posing a potential hindrance to its use in biodiesel production. Commonly, the lipid production enhanced in depletion condition which was not faced in the current study, as the wastewater enriched with several organic and inorganic compounds. It has been mentioned that certain inhibitors in wastewater may exist, which could impede culture performance with respect to growth and lipid storage. Nonetheless, this study serves as a proof of concept, and optimization of the process or the use of alternative wastewaters could lead to an increase in the fraction of lipids. Although the pigment content (expressed in contribution with other components) in biomass was merely 2.9%. These pigments are essential for the absorption of specific wavelengths throughout photosynthesis, and represent a natural green solution, which was increasingly sought by consumers due to their knowledge about the negative effects of chemical-based sources (Nagarajan et al. 2019).
In the point of the energy consideration, it is required to heat the potato juice to 80 °C for the protein coagulation process with protamylasse as a sidestream. The water is evaporated under vacuum and the needed electrical energy is supplied with a ventilator. The amount of energy needed is 2030 kJ per kg potato juice considering 10 times concentration and the enthalpy of water evaporation (2255 J g−1). The amount of energy needed for heating the AA process (1 kg diluted protamylasse from 10 to 55 °C) is much lower with 188 kJ kg−1 considering thermophilic conditions. This was found considering the specific heat capacity of water (4.18 J g−1 K−1). If evaporation is avoided by using potato juice directly for the AA process and correspondingly less dilution for the algal cultivation, 1974 kJ kg−1 potato juice can be saved. However, considering the required protein coagulation for protein production and storage problems of potato juice it is still most viable to do the evaporation process.
Conclusion
The cultivation approach and conditions employed on the microalgae C. sorokiniana were found to be effective in terms of nutrient removal from the protamylasse. The produced algal biomass with high content of protein (74%) generates a prospect for the production of nutritional supplements as well as ingredients for animal feeds. This production concept was enabled by pretreatment of the protamylasse by anaerobic acidification aiming at volatile fatty acid production, which could be metabolized by the microalgae during mixotrophic growth.
Data availability
All data generated or analyzed during this study are included in this published article and the supporting information.
References
Aniyikaiye TE, Oluseyi T, Odiyo JO, Edokpayi JN (2019) Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria. Int J Environ Res Public Health 16:1235
Asadi P, Rad HA, Qaderi F (2019) Comparison of Chlorella vulgaris and Chlorella sorokiniana pa.91 in post treatment of dairy wastewater treatment plant effluents. Environ Sci Pollut Res 26:29473–29489
Batista AP, Niccolai A, Fradinho P, Fragoso S, Bursic I, Rodolfi L, Biondi N, Tredici MR, Sousa I, Raymundo A (2017) Microalgae biomass as an alternative ingredient in cookies: sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res 26:161–171
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew Sustain Energy Rev 19:360–369
Chaudry S, Bahri PA, Moheimani NR (2015) Pathways of processing of wet microalgae for liquid fuel production: a critical review. Renew Sustain Energy Rev 52:1240–1250
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Bioethanol 26:131
Chia MA, Lombardi AT, da Graça Gama Melão M, Parrish CC (2015) Combined nitrogen limitation and cadmium stress stimulate total carbohydrates, lipids, protein and amino acid accumulation in Chlorella vulgaris (Trebouxiophyceae). Aquat Toxicol 160:87–95
Cui H, Ma H, Chen S, Yu J, Xu W, Zhu X, Gujar A, Ji C, Xue J, Zhang C, Li R (2020) Mitigating excessive ammonia nitrogen in chicken farm flushing wastewater by mixing strategy for nutrient removal and lipid accumulation in the green alga Chlorella sorokiniana. Bioresour Technol 303:122940
de Silva MET, Leal MA, de Oliveir Resende M, Martins MA, doe Reis Ciobra JS (2021) Scenedesmus obliquus protein concentrate: A sustainable alternative emulsifier for the food industry. Algal Res 59:102468
de Sousa SKD, Henrique IN, Brasil DP, Santos EC (2008) Anaerobic digestion and the denitrification in UASB reactor. J Urban Environ Eng 2:63–67
Do JM, Jo SW, Yeo HT, Shin DH, Oh H, Hong JW, Yoon H-S (2021) Biological treatment of reverse osmosis concentrate by microalgae cultivation and utilization of the resulting algal biomass. J Water Process Eng 42:102157
Downing TG, Sember CS, Gehringer MM, Leukes W (2005) Medium N: P ratios and specific growth rate comodulate microcystin and protein content in Microcystis aeruginosa PCC7806 and M. aeruginosa UV027. Microb Ecol 49:468–473
Elbahloul Y, Frey K, Sanders J, Steinbüchel A (2005) Protamylasse, a residual compound of industrial starch production, provides a suitable medium for large-scale cyanophycin production. Appl Environ Microbiol 71:7759–7767
Ende SSW, Noke A (2019) Heterotrophic microalgae production on food waste and by-products. J Appl Phycol 31:1565–1571
Fang C, Boe K, Angelidaki I (2011) Biogas production from potato-juice, a by-product from potato-starch processing, in upflow anaerobic sludge blanket (UASB) and expanded granular sludge bed (EGSB) reactors. Bioresour Technol 102:5734–5741
Fotidis IA, Wang H, Fiedel NR, Luo G, Karakashev DB, Angelidaki (2014) Bioaugmentation as a solution to increase methane production from an ammonia-rich substrate. Environ Sci Technol 48:7669–7676
Gautam P, Lal B, Panda BB, Bihari P, Chatterjee D, Singh T, Nayak PK, Nayak AK (2021) Alteration in agronomic practices to utilize rice fallows for higher system productivity and sustainability. Food Crops Res 260:108005
Georgacakis D, Sievers DM, Iannotti EL (1982) Buffer stability in manure digesters. Agric Wastes 4:427–441
Hamza RA, Iorhemen OT, Zaghloul MS, Tay JH (2018) Rapid formation and characterization of aerobic granules in pilot-scale sequential batch reactor for high-strength organic wastewater treatment. J Water Process Eng 22:27–33
He PJ, Mao B, Shen CM, Shao LM, Lee DJ, Chang JS (2013) Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour Technol 129:177–181
He ZW, Tang CC, Liu WZ, Ren YX, Guo ZC, Zhou AJ, Wang L, Yang CX, Eang AJ (2019) Enhanced short-chain fatty acids production from waste activated sludge with alkaline followed by potassium ferrate treatment. Bioresour Technol 289:121642
Ilavarasi A, Mubarakali D, Praveenkumar R, Baldev E, Thajuddin N (2011) Optimization of various growth media to freshwater microalgae for biomass production. Biotechnology 10:540–545
Li K, Liu Q, Fang F, Luo R, Lu Q, Zhou W, Huo S, Cheng P, Liu J, Addy M, Chen P, Chen D, Ruan R (2019) Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour Technol 291:121934
Li Y, Park SY, Zhu J (2011) Solid-state anaerobic digestion for methane production from organic waste. Renew Sustain Energy Rev 15:821–826
Lizzul AM, Hellier P, Purton S et al (2014) Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour Technol 151:12–18
Lois-Milevicich J, Casá N, Alvarez P, Mateucci R, Busto V, de Escalada M (2020) Chlorella vulgaris biomass production using brewery wastewater with high chemical oxygen demand. J Appl Phycol 32:2773–2783
Lukitawesa PRJ, Millati R, Sárvári-Horváth I, Taherzadeh MJ (2020) Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 11:39–52
Marcilhac C, Sialve B, Pourcher AM, Ziebal C, Bernet N, Béline F (2014) Digestate color and light intensity affect nutrient removal and competition phenomena in a microalgal-bacterial ecosystem. Water Res 64:278–287
Maurya R, Zhu X, Valverde-Pérez B, Ravi Kiran B, General T, Sharma S, Kumar Sharma A, Thomsen M, Venkata Mohan S, Mohanty K, Angelidaki I (2022) Advances in microalgal research for valorization of industrial wastewater. Bioresour Technol 343:126128
Mohamadnia S, Tavakoli O, Ali M (2021) Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 533:736074
Mohamadnia S, Tavakoli O, Faramarzi MA (2022a) Optimization of metabolic intermediates to enhance the production of fucoxanthin from Tisochrysis lutea. J Appl Phycol 34:1269–1279
Mohamadnia S, Tavakoli O, Faramarzi MA (2022b) Production of fucoxanthin from the microalga Tisochrysis lutea in the bubble column photobioreactor applying mass transfer coefficient. J Biotechnol 348:47–54
Nagarajan D, Kusmayadi A, Yen HW, Dong CD, Lee DJ, Chang JS (2019) Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour Technol 289:121718
Naseri A, Jacobsen C, Sejberg JJP, Pedersen TE, Larsen J, Hansen KM, Holdt SL (2020) Multi-extraction and quality of protein and carrageenan from commercial spinosum (Eucheuma denticulatum). Foods 9:1072
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2019) Light management technologies for increasing algal photobioreactor efficiency. Algal Res 39:101433
Pan M, Zhu X, Pan G, Angelidak I (2021) Integrated valorization system for simultaneous high strength organic wastewater treatment and astaxanthin production from Haematococcus pluvialis. Bioresour Technol 326:124761
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res 45:11–36
Procházka J, Dolejš P, MácA J, Dohányos M (2012) Stability and inhibition of anaerobic processes caused by insufficiency or excess of ammonia nitrogen. Appl Microbiol Biotechnol 93:439–447
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol 168:127–135
Rasdi NW, Qin JG (2015) Effect of N:P ratio on growth and chemical composition of Nannochloropsis oculata and Tisochrysis lutea. J Appl Phycol 27:2221–2230
Rasouli Z, Valverde-Pérez B, D’Este M, De Francisci D, Agelidaki I (2018) Nutrient recovery from industrial wastewater as single cell protein by a co-culture of green microalgae and methanotrophs. Biochem Eng J 134:129–135
Ren H, Zhu G, Ni J, Shen M, Show PL, Sun FF (2022) Enhanced photoautotrophic growth of Chlorella vulgaris in starch wastewater through photo-regulation strategy. Chemosphere 307:135533
Renuka N, Sood A, Prasanna R, Ahluwalia AS (2015) Phycoremediation of wastewaters: a synergistic approach using microalgae for bioremediation and biomass generation. Int J Environ Sci Technol 12:1443–1460
Rude K, Yothers C, Barzee TJ, Kutney S, Zhang R, Franz A (2022) Growth potential of microalgae on ammonia-rich anaerobic digester effluent for wastewater remediation. Algal Res 62:102613
Shim SJ, Hong ME, Chang WS, Sim SJ (2020) Repeated-batch production of omega-3 enriched biomass of Chlorella sorokiniana via calcium-induced homeoviscous adaptation. Bioresour Technol 303:122944
Van Wagenen J, Holdt SL, De Francisci D, Valverde-Pérez B, Plósz BG, Angelidaki I (2014) Microplate-based method for high-throughput screening of microalgae growth potential. Bioresour Technol 169:566–572
Wágner DS, Cazzaniga C, Steidl M, Dechesne A, Valverde-Pérez B, Plósz BG (2021) Optimal influent N-to-P ratio for stable microalgal cultivation in water treatment and nutrient recovery. Chemosphere 262
Xue C, Gao K, Qian P, Dong J, Gao Z, Liu Q, Chen B, Deng X (2021) Cultivation of Chlorella sorokiniana in a bubble-column bioreactor coupled with cooking cocoon wastewater treatment: Effects of initial cell density and aeration rate. Water Sci Technol 83:2615–2628
Yahampath Arachchige Don CDY, Babel S (2021) Effects of organic loading on bioelectricity and micro-algal biomass production in microbial fuel cells using synthetic wastewater. J Water Process Eng 39:101699
Yang M, Xue C, Li L, Gao Z, Liu Q, Qian P, Dong J, Guo K (2022) Design and performance of a low-cost microalgae culturing system for growing Chlorella sorokiniana on cooking cocoon wastewater. Algal Res 62:102607
Zhao X, Tang X, Wang Y (2009) Interactions between two species of marine bloom microalgae under controlled laboratory conditions: Heterosigma akashiwo and Karenia mikimotoi. Chin J Plant Ecol 33:958–965
Acknowledgements
KMC, Kartoffelmelcentralen Amba, 7330 Brande, Denmark is acknowledged for their input on the process and providing the protamylasse wastewater for the work.
Funding
Open access funding provided by Technical University of Denmark This project was financially supported by the projects InWAP and BlueBioChain granted by Innovation Fund Denmark with the grant numbers 8127-00007B and 1036-00003B, respectively. Additionally, the European Regional Development Fund (REACT-EU) is acknowledged for funding the project “Biosolutions Zealand”.
Author information
Authors and Affiliations
Contributions
Conception and design: SM, AT, PGI, IA (the letters represent the initials of the authors first and surnames letters); Conducting experiments: SM, AT, PGI, APM; Analysis and interpretation of the data: SM, AT, PGI, IA, BVP; Drafting of the article: SM, AT, APM; Critical revision of the article: IA, SM. All authors have read and approved the final document.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamadnia, S., Thygesen, A., Ghofrani-Isfahani, P. et al. Valorization of potato starch wastewater using anaerobic acidification coupled with Chlorella sorokiniana cultivation. J Appl Phycol 35, 2645–2658 (2023). https://doi.org/10.1007/s10811-023-03046-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03046-4