Abstract
The red alga Asparagopsis armata is an emerging aquaculture-target species due to its application as an antimethanogenic feed ingredient in ruminants, yet information on A. armata reproduction and cultivation is currently lacking. We therefore quantified the effects of temperature, irradiance, nutrients, and photoperiod, and addition of plant growth regulators (PGRs; indole-3-acetic acid, abscisic acid, 1-aminocyclopropane-1-carboxylic acid) on tetrasporogenesis in domesticated A. armata that had been maintained under controlled conditions (18 °C, 12 h light:12 h dark photoperiod) for 18 months prior to experimentation. Tetrasporogenesis was only induced at 5 and 15 µmol photons m−2 s−1 under an 8 h light:16 h dark photoperiod with 3.5 mg nitrogen L−1 and tetraspore release was 28-fold greater at 18 °C compared to 15 °C after 28 days of exposure. After 29 days, tetraspore release and germination rate both declined. All PGR treatments prevented tetrasporogenesis. This study is the first to provide the detail and framework necessary to enable A. armata hatchery development. We conclude that tetrasporogenesis was most likely induced in response to a significant reduction in photoperiod rather than as a result of replicating seasonal environmental conditions, and that temperature played a key role in determining reproductive output. With overall higher tetraspore release and a consistent germination rate of > 90%, we recommend exposing tetrasporophyte biomass to 18 °C, 15 µmol photons m−2 s−1 and 3.5 mg nitrogen L−1 under an 8 h L:16 h D photoperiod for up to 29 days to obtain a reliable supply of tetraspores for seeding onto ropes for transfer to the hatchery phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The red seaweeds Asparagopsis armata and Asparagopsis taxiformis are potent inhibitors of enteric methane due to their production and storage of the bioactive secondary metabolite bromoform (Kinley et al. 2016, 2020; Li et al. 2016; Machado et al. 2016, 2018; Roque et al. 2021). As a result, there is high interest to commercially cultivate these seaweeds at scale to supply the beef and dairy industries with biomass for inclusion in anti-methanogenic feeds (Duarte et al. 2017). However, before wide-scale adoption of Asparagopsis becomes possible, further research is required to develop techniques for large-scale commercialisation, product application technologies, and management strategies to ensure the long-term safety and efficacy of Asparagopsis in animal production systems (Vijn et al. 2020; Pandey et al. 2021; Glasson et al. 2022).
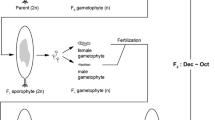
The commercialisation of Asparagopsis requires a continuous supply of high quality biomass that will need to be provided through efficient and sustainable aquaculture (Vijn et al. 2020). As such, there are three approaches that can be used to farm Asparagopsis, namely: a) land-based aquaculture of the tetrasporophyte in tanks (Mata et al. 2010), b) mariculture of wild-harvest gametophyte by seeding ropes for grow-out (Kraan and Barrington 2005; Wright et al. 2022), and c) closed life-cycle cultivation of gametophytes from tetraspores for mariculture on ropes (Fig. 1). Closed-life cycle cultivation is a desirable method as it enables the year-round production of gametophytes via the induced reproduction of tetrasporophytes, although the complex life cycle of Asparagopsis (Fig. 1) makes it challenging to close all the stages of the life cycle under aquaculture. This approach will also enable future strain selection and selective breeding for the production of high quality Asparagopsis containing a high content of bromoform (the primary bioactive component in Asparagopsis (Machado et al. 2016; Glasson et al. 2022), thereby reducing the dosage of Asparagopsis required to achieve an optimal mitigation effect (Li et al. 2016; Charrier et al. 2017; Roque et al. 2019, 2021; Kinley et al. 2020).
Schematic diagram of the life cycle of Asparagopsis armata has a triphasic life cycle, alternating between a terete gametophyte (n), a microscopic carposporophyte (2n), and filamentous tetrasporophyte (2n) phase. Gametophytes are dioecious, with males producing spermatangia and females producing carpogonia. After fertilisation, the carposporophytes develop on the female thallus. Carposporophytes produce carposporangia, which release carpospores that germinate into tetrasporophytes. Tetrasporophytes (also referred to as individual ‘pom poms’) develop tetrasporangia, which release tetraspores that germinate into gametophytes (Bonin & Hawkes 1987). Original pencil drawings, assembly and edits in Adobe Photoshop and Illustrator by Maro Guy. Drawings are not to scale
Successful seaweed aquaculture requires a high degree of control over the external factors regulating seaweed reproduction, such as irradiance, temperature, photoperiod, pH, and nutrient concentration (García-Jiménez and Robaina 2015; Charrier et al. 2017; Liu et al. 2017). Identifying the optimum conditions to induce tetrasporogenesis (i.e., the mass formation of tetrasporangia) and the release, settlement, and germination of subsequent tetraspores into juvenile gametophytes are pivotal steps in establishing closed-life cycle aquaculture of Asparagopsis as these will enable the development of commercial hatcheries for generating sufficient and sustainable biomass supply to meet future demands (Pang and Lüning 2004; Charrier et al. 2017). Temperature, photoperiod, and nutrient concentration are critical factors for inducing tetrasporogenesis in Asparagopsis, and tetrasporogenesis has only been induced under short photoperiods (Oza 1977; Bonin and Hawkes 1987; Guiry and Dawes 1992). For example, when cultured at 17 °C, the critical photoperiod for inducing tetrasporogenesis in Irish and Australian strains of A. armata tetrasporophytes ranged between 8 h L:16 h D (Light:Dark) – 9 h L:15 h D (Guiry and Dawes 1992). Similarly, tetrasporogenesis occurred when cultured at 15 °C under low nutrient concentrations (i.e. nitrate/phosphate deficient) and at photoperiods ranging between 6 h L:18 h D – 8 h L:16 h D in French strains of A. armata (Oza 1977). However, no method with sufficient detail surrounding the timing and practical setup for inducing tetrasporogenesis or optimising the release, settlement and germination of tetraspores has been published.
There is accumulating evidence that the application of plant growth regulators (PGRs) can provide benefits for aquaculture by accelerating and/or enhancing seaweed reproduction (Sacramento et al. 2007; Stirk and Van Staden 2014; Pilar et al. 2016; Garcia-Jimenez and Robaina 2017; Liu et al. 2017). For example, the exogenous application of methyl jasmonate (100 µM) to Grateloupia imbricata reduced the cystocarp maturation period to 48 h compared to the typical > 3-week period and increased the number of cystocarps 7.5-fold compared to controls (Pilar et al. 2016). Multiple studies have also detected increases in the production of indole-3-acetic acid (IAA), abscisic acid (ABA), and 1-aminocyclopropane-1-carboxylic acid (ACC) in fertile thalli compared to infertile thalli in several species of seaweed (Nimura and Mizuta 2002; Kai et al. 2006; García-Jiménez and Robaina 2012, 2015; Uji et al. 2020), indicating that these PGRs may play a role in regulating seaweed reproduction. There is significant potential for PGRs to improve the efficiency of A. armata grown in hatcheries by reducing the induction period for tetrasporogenesis, which would drastically improve the production of tetraspores over the same time scale, resulting in reduced overall costs of hatchery production (Watson and Dring 2011; Taelman et al. 2015).
The aim of this study was therefore to identify the optimal cultivation conditions for inducing and controlling tetrasporogenesis in the anti-methanogenic red seaweed A. armata in New Zealand. This will provide a baseline method for obtaining a reliable supply of A. armata tetraspores to facilitate the development of sustainable A. armata aquaculture. The specific objectives of this study were to: (1) determine the effect of temperature, irradiance, nutrient concentration, and photoperiod on the induction of tetrasporogenesis under controlled laboratory conditions; (2) quantify the number of tetraspores released and germination rate of released tetraspores under successful inducing conditions; (3) determine the effect of selected PGRs on tetrasporogenesis with the target of accelerating tetrasporogenesis; and (4) regulate the induction of tetrasporogenesis (i.e., “switching” reproduction off and on) under controlled laboratory conditions.
Materials and methods
Sample collection and tetrasporophyte production
Cystocarpic Asparagopsis armata (approximately 20 individuals) were haphazardly collected from natural populations (Ministry for Primary Industries Special Permit number 742) at 2 – 3 m depth by snorkelling in Matheson Bay, Leigh, New Zealand (36.31°S, 174.80°E) in August 2020. Samples were transported in buckets filled with seawater collected at the site to the laboratory. The collected specimens were transferred to nutrient enriched (3.5 mg nitrogen (N) L−1 and 0.3 mg phosphorus (P) L−1 (F/8), Varicon Aqua, Cell-Hi F2P) filtered seawater upon arrival to the laboratory and maintained in a temperature and light controlled room (18 °C, ~ 15 µmol photons m−2 s−1, 12 h L:12 h D; spring/autumn conditions at Matheson Bay). Within a week, carpospores were released that had germinated into tetrasporophytes. Tetrasporophytes were scraped from the bottom of the bucket where they had settled and transferred into a new 2 L white plastic bucket filled with autoclaved-filtered seawater (AFSW) with nutrients added at F/8. Germanium dioxide (GeO2) was added at a concentration of 2.5 mL L−1 to inhibit diatom growth. Tetrasporophytes were scaled up and maintained over an 18-month period under the same conditions described above with weekly water changes and were well acclimated to pre-experimental culture conditions. This biomass was used in the 4 succeeding experiments:
-
(a)
Induction of tetrasporogenesis
-
(b)
Enhancing tetrasporogenesis
-
(c)
Use of plant growth regulators
-
(d)
Controlling repeated cycles of tetrasporogenesis
Experimental overview
The present study was divided into four experiments:
In the first experiment ((a) induction of tetrasporogenesis, Fig. 2a), A. armata tetrasporophyte filaments were exposed to different combinations of temperature, irradiance, and nutrient concentration to determine the effect of these environmental drivers on the induction of tetrasporogenesis under controlled laboratory conditions. Several treatment combinations resulted in the induction of tetrasporogenesis during this experiment but only at 15 °C (i.e., no treatments that included 11 or 13 °C resulted in tetrasporogenesis); therefore, in the second experiment ((b) enhancing tetrasporogenesis, Fig. 2b), the conditions were further refined by including photoperiod and adding a higher temperature treatment. Here, the number of released and settled tetraspores (hereafter referred to as released tetraspores) and the germination rate of released tetraspores were quantified under each set of conditions to identify an optimal set of conditions for inducing tetrasporogenesis. In the third experiment ((c) use of plant growth regulators), tetrasporophyte filaments exposed to the optimal set of conditions for inducing tetrasporogenesis were treated with selected PGRs at a range of concentrations to assess their effect on the induction of tetrasporogenesis with a target of shortening the required hatchery period. In the fourth experiment ((d) controlling repeated cycles of tetrasporogenesis), filaments were switched between the optimal set of conditions for inducing tetrasporogenesis and a set of conditions targeted at ceasing tetrasporogenesis to assess whether tetrasporogenesis can be ceased and restarted in the same biomass or if biomass is ‘spent’ following mass-production of tetraspores. Water changes were carried out weekly during all experiments.
a Experimental design for the first experiment testing the effect of temperature, irradiance, and nutrient media (F/8 = 3.5 mg N L−1 and 0.3 mg P L−1, F/20 = 1.4 mg N L−1 and 0.1 mg P L.−1) on the induction of tetrasporogenesis (n = 3). Photoperiod (L:D) was maintained at 8 h L:16 h D throughout the experiment. (b) Experimental design for the second experiment testing the effect of temperature, irradiance, and photoperiod on the induction of tetrasporogenesis with the aim of enhancing tetrasporogenesis (n = 5)
a) Induction of tetrasporogenesis
Twenty individual tetrasporophytes from the stock cultures were cut into 3 – 4 mm length filaments using dissecting scissors to obtain a homogenous mass of cuttings. Subsamples of approximately 40 – 50 filaments (~ 10 mg fresh weight (FW)) per replicate were transferred into individual Petri dishes (90 × 20 mm, LabServ, LBS60016) filled with 50 mL of AFSW with nutrients (Varicon Aqua, Cell-Hi F2P) added at concentrations of F/8 (3.5 mg N L−1 and 0.3 mg P L−1) and F/20 (1.4 mg N L−1 and 0.1 mg P L−1). Dishes were placed in environmental control cabinets (Panasonic; MLR-352) set at 11, 13, and 15 °C and light setting 1 (LS1) with a photoperiod of 8 h L:16 h D based on previously published papers (Oza 1977; Guiry and Dawes 1992). All dishes were placed at the back of the light source where light measurements (LI-COR; LI-1500) confirmed an average irradiance of 15 μmol photons m−2 s−1. The 5 μmol photons m−2 s−1 treatment was achieved by covering dishes with a white plastic tray. This resulted in 12 treatment combinations in a fully factorial design (n = 3 for each treatment, Fig. 2a). Treatments were maintained under experimental conditions for 42 days. All treatments were visually inspected weekly for the presence of tetrasporangia by examining 30 filaments from each dish under a stereomicroscope (Olympus SZX2-ILLTQ), and the presence/absence of tetrasporangia for each treatment was recorded.
b) Enhancing tetrasporogenesis
Tetrasporangia were only formed at 15 °C with F/8 treatments exposed to both 5 and 15 μmol photons m−2 s−1 during the first experiment. Therefore, we used these conditions as the baseline for the second experiment, which was a factorial experiment investigating the effect of temperature (15 and 18 °C), irradiance (5 and 15 μmol photons m−2 s−1), and photoperiod (8 H L: 16 H D and 12 H L: 12 H D) with F/8 resulting in eight treatment combinations (n = 5 for each treatment, Fig. 2b). A homogenous mass of tetrasporophyte filaments was prepared and subsamples were transferred into Petri dishes as described in (a). Nutrients were added at F/8. Dishes were placed in environmental control cabinets (Bio-strategy; MLR-352) set at temperatures of 15 and 18 °C and LS1 with photoperiods of 12 h L:12 h D and 8 h L:16 h D. Irradiances of 5 and 15 μmol photons m−2 s−1 were achieved as described in (a). The experiment was carried out in two parts, described below.
For part one of the experiment (day 0 – 27), all treatments were maintained under experimental conditions for 27 days (based on temperature, light and nutrient conditions that led to the successful induction of tetrasporogenesis after 21 days during the first experiment (a)) to determine which treatment combination produced the highest number of released tetraspores over the shortest period of time and with the highest germination rate of released tetraspores. After 7 days, treatments were visually inspected daily for the presence/absence of tetrasporangia by examining 30 filaments from each dish under a stereomicroscope and the presence/absence of tetrasporangia for each treatment was recorded. For treatments in which tetrasporogenesis was successfully induced, the number of tetraspores released and germination rate of released tetraspores were quantified daily by counting the total number of newly released (ungerminated) and germinated tetraspores settled at the bottom of each dish using a stereomicroscope, as the first division of the germinating tetraspore is evident after 24 h (Fig. 3). The biomass was kept in the same dish throughout this part of the experiment.
For part two of the experiment (day 28 – 52), only the most successful treatment combination (i.e. that in which filaments produced the highest number of released tetraspores over the shortest period of time and with the best germination rate of released tetraspores) after 27 days was continued to determine the peak time period for tetraspore release and the cut-off point for tetrasporogenesis under these conditions. Due to the high total number of tetraspores released per day (> 500 day−1 per dish) during part one of the experiment, the method for quantifying tetraspore release and germination rate was adjusted as follows: each day, the entire contents (biomass and water) of each dish were transferred into a new dish and tetraspore release was quantified by counting the number of released tetraspores at the bottom of each original dish. New nutrient medium was added back into the dishes which were transferred back to their respective culture cabinets. Germination rate was then quantified by counting the number of germinated tetraspores in each original dish three days post-release. Treatments were maintained under experimental conditions for a total of 52 days.
(c) Use of plant growth regulators
A homogenous mass of tetrasporophyte filaments was prepared and subsamples were transferred into Petri dishes as described in(a). Three PGRs—(a) indole-3-acetic acid (IAA), (b) abscisic acid (ABA), and (c) 1-aminocyclopropane-1-carboxylic acid (ACC)—were applied at three concentrations: 0.1 μM, 1.0 μM, and 10 μM (n = 3 for each treatment), and a control with no PGRs added was also included (n = 3). PGR stock solutions were prepared using reverse osmosis (RO) treated water at concentrations of 17.5 mg (100 mL)−1, 10.1 mg (100 mL)−1, and 26.43 mg mL−1 for IAA, ABA, and ACC, respectively. IAA and ABA were dissolved in ethanol (EtOH) and dimethyl sulfoxide (DMSO), respectively, as carriers due to their low solubility in water. Solvent controls were previously tested with no effect detected and were therefore not included in the run. PGR stock solutions were added to each dish according to the three treatment concentrations and topped up to 50 mL with AFSW and nutrients added at F/8. Dishes were placed in an environmental control cabinet (Panasonic; MLR-352) set at 18 °C, 15 μmol photons m−2 s−1 (LS1), and an 8 h L:16 h D photoperiod. All treatments were visually inspected every second day for the presence of tetrasporangia by examining 30 filaments from each dish under a stereomicroscope and the presence/absence of tetrasporangia for each treatment was recorded. Water changes were carried out with PGRs added as described above. As the aim of this experiment was to determine whether tetrasporogenesis can be accelerated to shorten the required hatchery period, the experiment was run for a total of 14 days based on the successful induction of tetrasporogenesis after 14 days under these conditions during the second experiment (b).
(d) Controlling repeated cycles of tetrasporogenesis
A pilot experiment using excess reproductive tissue grown under the optimal conditions for inducing tetrasporogenesis (i.e. 18 °C, 15 μmol photons m−2 s−1, 8 h L: 16 h D, F/8) in the second experiment (b)was conducted to identify conditions to cease tetrasporogenesis. On day 30 and 42 of the second experiment, approximately 3 – 4 filaments from each replicate Petri dish were combined and transferred into separate Petri dishes (90 × 20 mm) filled to 50 mL with AFSW and incubated under conditions selected to inhibit tetrasporogenesis (n = 1, Fig. 4a). The filaments transferred on day 30 were grown at 18 °C, 15 μmol photons m−2 s−1, 12 h L: 12 h D, and with nutrients added at F/8, whereas the filaments transferred on day 42 were exposed to 20 °C, 5 μmol photons m−2 s−1, 15 h L:9 H D, and with nutrients added at F/4 (7.0 mg N L−1). All treatments were visually inspected every 2 – 3 days for the presence of tetrasporangia by examining all filaments from each dish under a stereomicroscope and the presence/absence of tetrasporangia for each treatment was recorded. Both treatments were maintained under experimental conditions for 16 days, or until tetrasporogenesis had ceased. Since tetrasporangia only ceased in 20 °C, 5 μmol photons m−2 s−1, 15 h L:9 H D, F/4 treatments (non-inducing conditions), these conditions were tested with new stock culture biomass as described below to confirm their effects on tetrasporogenesis.
Experimental flow for (a) pilot trial and (b) the fourth experiment where tetrasporophytes were exposed to different sets of conditions targeted at either inducing (condition I; 18 °C, 15 μmol photons m−2 s−1, 8 h L: 16 h D, F/8 (3.5 mg N L−1), ceasing (condition II; 20 °C, 5 μmol photons m−2 s−1, 15 h L:9 H D, F/4 (7.0 mg N L−1) and condition III; 18 °C, 15 μmol photons m−2 s−1, 12 h L: 12 h D, F/8), or re-starting (condition I) tetrasporogenesis. Days indicate the time under each set of conditions. Box colour indicates whether biomass was reproductive (grey) or non-reproductive (white) during each stage of the experiment
A homogenous mass of tetrasporophyte filaments was prepared and subsamples were transferred into Petri dishes as described in (a). Nutrients were added at F/8 and dishes were placed in an environmental control cabinet set at 18 °C, 15 μmol photons m−2 s−1 (LS1), and a photoperiod of 8 h L:16 h D (inducing conditions, n = 5, Fig. 4b). Dishes were visually inspected to confirm the presence of tetrasporangia after 14 days as described in (c). Treatments were maintained under inducing conditions for a further 16 days, and then visually inspected to confirm tetrasporogenesis was still occurring. Cabinet settings were then changed to 20 °C and 5 μmol photons m−2 s−1, and a photoperiod of 15 h L:9 h D, and the nutrient concentration was increased to F/4 (non-inducing conditions). Treatments were visually inspected every second day for the presence/absence of tetrasporangia as described previously and were maintained under these experimental conditions for 14 days, or until tetrasporogenesis had ceased. Once tetrasporangia were no longer present inside filaments, cabinet settings were changed back to inducing conditions and treatments were visually inspected weekly for the presence of tetrasporangia. Treatments were maintained under inducing conditions for 21 days, or until tetrasporangia were formed, at which point the experiment ended.
Statistical analyses
Three-factor analysis of variance (ANOVA) was used to compare the effect of temperature, light, and photoperiod (fixed factors) on the number of tetraspores released per day. As tetrasporogenesis is a prolonged process, analyses on tetraspore release were carried out using data that had been averaged across day 14 – 20 (week 3) and day 21 – 27 (week 4) for each treatment; data for these time periods were analysed separately to identify the best treatment combination at each time period, rather than to detect significant differences over time. Data were log(x + 1) transformed to improve homogeneity of variances if Levene’s test for homogeneity of variances was significant. When ANOVA detected significant differences between means (α = 0.01), Tukeys HSD post-hoc tests were used to compare the means of the treatment groups. Eta-squared (%) ɳ2 = SSfactor / SStotal × 100; with SSfactor being the sum of squares of a particular factor and SStotal being the total sum of squares, was calculated to determine the proportion of the total variation in the number of tetraspores released that was associated with each factor (temperature, light, and photoperiod) (Richardson 2011). Formal statistical analyses were not carried out for germination rate because including all replicates shows a decline in germination rate on days where some replicates had no released tetraspores. Consequently, only including replicates where tetraspores were released would result in an unbalanced design with low statistical power due to a low number of replicates. All statistical analyses were conducted using RStudio (version 3.6.1). All data are reported as mean ± standard error (SE); n = 3 for experiment (a) and (c), n = 5 for experiment (b) and (d).
Results
Induction of tetrasporogenesis
Tetrasporangia were formed only in 15 °C and F/8 treatments exposed to either 5 (2/3 replicates) or 15 µmol photons m−2 s−1 (3/3 replicates) and were first visible from day 21 of the experiment (Table 1). Tetrasporangia continued to form on day 28 and 35, and by day 28, both treatments had released tetraspores that had successfully settled and germinated into juvenile gametophytes (Table 1). No tetrasporangia were formed on day 42. No tetrasporangia were formed in 11 °C and 13 °C or F/20 treatments exposed to either irradiance at any point during the experiment.
Enhancing tetrasporogenesis
Tetrasporangia were formed only in 18 and 15 °C treatments exposed to 15 µmol photons m−2 s−1 and an 8 h L:16 h D photoperiod and first began to form on day 14 and 17 of the experiment, respectively. The number of tetraspores released per day (tetraspores day−1) differed significantly among temperature treatments, but the effects were not consistent among photoperiod or light treatments (Table 2). Tetraspore release was ninefold and 28-fold greater for 18 °C treatments compared to 15 °C treatments during day 14 – 20 and day 21 – 27 of the experiment, respectively (P = 0.007, Fig. 5). Tetraspore release was higher during day 21 – 27 of the experiment for both temperature treatments, with an average of 541 ± 136 (18 °C) and 20 ± 7 (15 °C) tetraspores day−1 during day 21 – 27 compared to an average of 56 ± 34 (18 °C) and 6 ± 5 (15 °C) tetraspores day−1 during day 14 – 20, and there were generally large variations in tetraspore release occurring between replicates of the same treatment (Fig. 5). Approximately 14% of the variation in tetraspore release was due to the interaction between light and photoperiod (LxL:D), as well as light and photoperiod as individual factors, whereas approximately 10% of the variation was due the remaining interactions (TxL, TxL:D, TxLxL:D) and temperature as an individual factor (Table 2). The germination rate of released tetraspores during day 20 – 14 and day 21 – 27 ranged between 94 ± 6 – 97 ± 3% for both treatments (Fig. 6). No tetrasporangia were formed in treatments exposed to 5 µmol photons m−2 s−1 or a 12 h L:12 h D photoperiod.
Mean (± S.E) tetraspore release (day−1) 14 – 20 days and 21 – 27 days after initial exposure to different treatments ((b) enhancing tetrasporogenesis) (n = 5). Treatment names correspond to the given temperature (T, °C), light (L, µmol photons m−2 s−1), and photoperiod (L:D, h) conditions for each treatment. Pre-experimental culture conditions (18 T; 15 L; 12L: 12D) were used as a control
Mean (± S.E) germination rate (%) of released tetraspores (i.e., tetraspores germinated within 24 h of release) 14 – 20 days and 21 – 27 days after initial exposure to different treatments ((b) enhancing tetrasporogenesis) (n = 5). Treatment names correspond to the given temperature (T, °C), light (L, µmol photons m−2 s−1), and photoperiod (L:D, h) conditions for each treatment
Tetrasporophyte filaments exposed to 18 °C, 15 µmol photons m−2 s−1, and an 8 h L:16 h D photoperiod produced the highest number of released tetraspores during day 14 – 20 and day 21 – 27 while maintaining a germination rate of > 90%; therefore, these filaments were kept under the same treatment conditions for a further 25 days to continue assessing tetraspore release and germination rate. There were two peaks in tetraspore release under these conditions throughout the whole experimental period (Fig. 7a). The peaks occurred from day 27 – 29 (peak one) and day 49 – 50 (peak two) and were similar in size, with averages of 859 ± 173 and 757 ± 128 tetraspores day−1 respectively, with large variations in tetraspore release occurring between replicates of the same treatment (Fig. 7a). In contrast, the germination rate of released tetraspores was notably higher during peak one with an average of 92 ± 4% across peak one, compared to an average of 44 ± 8% across peak two, and was overall more consistent between samples of the same treatment (Fig. 7b). Tetrasporangia continued to form at the end of the experiment (day 52).
Mean (± S.E) (a) tetraspore release (day−1 per dish) and (b) germination rate (%) of released tetraspores (i.e. tetraspores germinated within 24 h of release from 18 °C, 15 µmol photons m−2 s.−1, and 8 h L:16 h D treatments from the onset of tetraspore release (D14) over a 52-day period ((b) enhancing tetrasporogenesis) (n = 5)
Plant growth regulators
Tetrasporangia were not observed in the seaweeds treated with PGRs after 14 days. As observed previously in the enhancing tetrasporogenesis experiment, tetrasporangia were formed in the 18 °C, 15 µmol photons m−2 s−1, and 8 h L:16 h D (control conditions with no PGRs added) treatment (3/3 replicates) after 14 days.
Controlling repeated cycles of tetrasporogenesis
In pilot trials (n = 1), tetrasporangia were present in filaments exposed to inducing conditions (18 °C, 15 µmol photons m−2 s−1, 12 h L:12 h D, and F/8) 14 days from the start of tetraspore formation. This was previously demonstrated to be peak tetraspore release before a decline was detected. Once transferred to non-inducing conditions (20 °C, 5 μmol photons m−2 s−1, 15 h L:9 H D, and F/4), tetrasporogenesis ceased after eight days.
In the replicated experiment (n = 5), tetrasporangia were present in filaments exposed to inducing conditions after 14 days and 14 days from the start of tetraspore formation. After 12 days of exposure to non-inducing conditions, tetrasporangia were no longer present in filaments. After 14 days of exposure back to inducing conditions, tetrasporangia were present again in filaments. These results were consistent in all five replicates.
Discussion
In this study we successfully induced tetrasporogenesis and the release and germination of tetraspores in domesticated A. armata tetrasporophytes that had been acclimated at 18 °C, ~ 15 µmol photons m−2 s−1, and 12 h L:12 h D for 18 months prior to experimentation. Reproductive processes in seaweeds are typically controlled by one or more environmental factors (Liu et al. 2017; de Bettignies et al. 2018) which can be manipulated to induce and optimise reproduction under controlled laboratory conditions for aquaculture purposes (Charrier et al. 2017). Previous studies assessing the effects of environmental factors on tetrasporogenesis in A. armata (Oza 1977; Lüning and Dieck 1989; Guiry and Dawes 1992) were carried out from an ecological standpoint, rather than with the purpose of informing and developing farming techniques, and thus lack the detail necessary for applying these methods at the scale required for commercial aquaculture. This study presents a quantitative analysis of the effects of temperature, irradiance, nutrient concentration, and photoperiod on the induction of tetrasporogenesis in A. armata tetrasporophytes and provides a foundational method for obtaining a high and reliable supply of tetraspores that can be upscaled to facilitate commercial hatchery development. There were clear interactive effects of light and photoperiod on tetrasporogenesis, and manipulating temperature was key to optimising this process. Based on our findings, the proposed method for optimising the production of tetraspores and their germination into juvenile gametophytes to maximise the number of tetraspores for seeding onto ropes for mass-scale seaweed cultivation is to expose tetrasporophytes to 18 °C, 15 µmol photons m−2 s−1 and F/8 (3.5 mg N L−1) under an 8 h L:16 h D photoperiod for up to 29 days.
Our findings demonstrate that a specific combination of temperature, irradiance, photoperiod, and nutrients is required to induce tetrasporogenesis in A. armata. A photoperiod of 8 h L:16 h D was critical for inducing tetrasporogenesis in our New Zealand strain of A. armata, but only at temperatures of 15 and 18 °C and a nutrient concentration of F/8 (3.5 mg N L−1). This was similar to previous studies where tetrasporogenesis was only induced under short photoperiods in combination with relatively narrow temperature bands that differed between strains (Oza 1977; Lüning and Dieck 1989; Guiry and Dawes 1992) (Table 3). The optimal conditions that induced tetrasporogenesis in our New Zealand strain, as well as several previously assessed strains (Oza 1977; Guiry and Dawes 1992), are similar to the natural spring/autumn temperature and winter photoperiod at the collection locations for these strains (Tables 3 and 4). These temperature and photoperiod conditions do not occur simultaneously during any season for the majority of assessed strains (Table 3). Furthermore, the inducing photoperiod identified in this study (8 h L:16 h D) does not naturally occur at the collection location of our strain (Table 4), suggesting that tetrasporogenesis was induced by stress induction through a significant reduction in photoperiod, rather than by replicating natural reproductive environmental conditions. Moreover, photoperiod was the only factor changed between the conditions that resulted in optimal tetraspore release in the second experiment (enhancing tetrasporogenesis) and the maintenance conditions of the stock cultures (18 °C, ~ 15 µmol photons m−2 s−1, 12 h L:12 h D) used to conduct the experiment. This conclusion is also supported by the fact that tetrasporogenesis is first induced in natural populations in March (austral autumn) when the ambient conditions are markedly different from those which induced tetrasporogenesis in this study (Tables 3 and 4) (Bonin and Hawkes 1987), although such high temperatures were not tested in the current study.
Tetrasporogenesis was optimal at 18 °C, with both tetraspore release and germination rate significantly higher than at 15 °C, whereas temperatures below 15 °C (11 and 13 °C) did not result in tetrasporogenesis, similar to previous studies (Oza 1977; Lüning and Dieck 1989; Guiry and Dawes 1992). While tetrasporogenesis occurred at 5 µmol photons m−2 s−1 during the first experiment (induction of tetrasporogenesis), there was higher variability between replicates than at 15 µmol photons m−2 s−1 and tetrasporogenesis did not occur at all at 5 µmol photons m−2 s−1 during the second experiment. Preliminary experiments with higher temperature and light conditions resulted in more bleached and fouled filaments. An irradiance of 15 µmol photons m−2 s−1 is therefore recommended as the minimum amount of light required for reliable induction. Nutrient depletion (~ 1.4 mg N L−1) prevented tetrasporogenesis under all conditions tested during the first experiment, which contrasts with a previous study reporting the induction of tetrasporogenesis under these same conditions for French strains of A. armata (Oza 1977) (Table 3). Conversely, nutrient concentration had no effect on the induction of tetrasporogenesis in Italian strains (Guiry and Dawes 1992) (Table 3). Variation in the effects of environmental factors on tetrasporogenesis are expected across geographically isolated strains of seaweed due to the reproductive synchrony of seaweeds with their surrounding environment (Ims 1990; Rule et al. 2013). Moreover, such variation may also occur across geographically separated populations of the same strain, or between co-existing strains as a result of underlying genetic differences (Mata et al. 2017; Jansen et al. 2022). The samples used in the present study were most likely of the L1B lineage, which is part of one of the two lineages (L1B and L2B within two cryptic clades L1 and L2) of A. armata found within New Zealand (Preuss et al. 2022). L1B is widespread throughout New Zealand, whereas L2B is currently found only in the south of the North Island to Stewart Island, where it has been found to co-exist with L1B in several locations (Preuss et al. 2022). Future work could look to expose A. armata tetrasporophytes from co-occurring L1B and L2B strains, as well as different populations of the L1B strain, to the optimal conditions identified in this study to further understanding of the effects of genetic versus environmental factors on the induction of tetrasporogenesis in A. armata.
The method reported in the present study significantly reduced the time required for induction compared to previous studies where tetrasporangia were induced after 5 – 8 weeks of exposure to experimental conditions (Oza 1977; Guiry and Dawes 1992). This is of critical importance for the viability of seeding at scale, as nursery and hatchery periods typically contribute a substantial proportion of ongoing operational costs (Watson and Dring 2011; Werner and Dring 2011; Taelman et al. 2015). There is also further potential for cost reduction through accelerated and enhanced reproduction with the use of PGRs. For example, sorus formation of the kelp Saccharina japonica was accelerated by two weeks with the application of ABA (10 µM) (Nimura and Mizuta 2002), while carpospore release in the red seaweed Pyropia yezoensis was significantly enhanced with the application of the ethylene precursor ACC (50 µM) (Uji et al. 2020). Contrary to these studies, there was no positive effect of PGRs on tetrasporogenesis in this study. One possible explanation for this was that one or more of these PGRs caused a shift in metabolism from reproduction to growth, as evinced by the suppression of sorus formation alongside increased growth in S. japonica with the addition of IAA (10 µM) (Kai et al. 2006). However, growth was not quantified here to enable further discussion in this regard. Little is known about the effects of PGRs specifically on tetrasporogenesis, with research concentrated on the effect of ethylene in Pterocladiella capillacea where exposure to ethylene (30 min) increased the number of tetrasporangial branches by nearly 200-fold compared to controls (García-Jiménez and Robaina 2012). Omics studies such as internal analyses of seaweed PGR contents from maintenance conditions through to a full reproductive cycle would facilitate the prospective application of PGRs for improving the efficiency of hatchery production.
Most cultivated seaweeds, including kelps and nori (Porphyra and Pyropia), undergo instant mass spore release where a high number of spores is immediately available for seeding at the onset of spore release (Redmond et al. 2013). Conversely, tetrasporogenesis in A. armata is a continuous process with variable sporulation in which there are evident peaks in tetraspore release. The method for seeding tetraspores will need to account for this. The methods reported here are suitable for obtaining a continuous high supply of tetraspores at the scale required for commercial hatchery development. Based on this study where ~ 10 mg FW of tetrasporophyte biomass was used per dish, an estimated > 10 million tetraspores per 100 g FW of tetrasporophyte biomass could be obtained for seeding over a 15-day period from day 14 at the onset of tetrasporogenesis to day 29 at the end of the first peak. However, this method would result in juvenile gametophytes at slightly different stages of development, which may pose challenges if there are marked differences in the requirements of early stage hatchery conditions. Another option could be to carry out seeding only at day 27 – 29 during the first peak, resulting in an estimated 7.6 – 8.6 million tetraspores per 100 g FW of tetrasporophyte biomass. The second peak in tetraspore release (day 49 – 50) was lower in both the number and germination rate of released tetraspores, and there was higher variability between replicates compared to the first peak. For these reasons, it is not recommended for seeding to be carried out during the second peak.
For the first time, we showed that tetrasporogenesis can be ceased on demand by exposing tetrasporophytes to an extended photoperiod of 15 h L:9 h D, alongside a decrease in irradiance (5 µmol photons m−2 s−1) and increase in temperature (20 °C) and nutrient concentration (7.0 mg N L−1), and then induced again by placing the same tetrasporophytes back under inducing conditions. This would prove useful in situations where tetrasporophyte biomass is limited. The ability to maintain and re-use previously-induced broodstock would also increase management options and be beneficial for research purposes (Jeliani et al. 2018). Additionally, as the second peak in tetrasporogenesis is less reproductive, broodstock may need to recover after being induced for seeding to reduce the risk of contamination that arises from increased time under seeding conditions (Mooney-McAuley et al. 2016). Whether repeatedly inducing tetrasporogenesis in the same biomass affects reproductive output and/or germination rate would need to be addressed in future work to assess whether this is a viable approach. Nevertheless, this work serves as a baseline for the process of undertaking repeated cycles of induced tetrasporogenesis to obtain A. armata tetraspores for seeding.
Conclusion
This study is the first to fundamentally optimise tetrasporogenesis and provide the detail and framework necessary for enabling A. armata hatchery development. We provide a baseline method for inducing tetrasporogenesis in A. armata and demonstrate that this process can be controlled through the manipulation of specific environmental parameters. Tetrasporogenesis was induced after 14 days when tetrasporophytes were exposed to an 8 h L:16 h D photoperiod with an irradiance of 15 µmol photons m−2 s−1 and nutrients added at a concentration of 3.5 mg N L−1 (F/8). Increasing the temperature from 15 to 18 °C resulted in a mass increase in tetraspore release which peaked after a total of 27 – 29 days of exposure to inducing conditions. In contrast, exposing tetrasporophytes to 11 and 13 °C under an 8 h L:16 h D photoperiod with lower light intensities and nutrient concentrations did not result in the induction of tetrasporogenesis. We conclude that tetrasporogenesis was most likely induced through stress in the form of a significant reduction in photoperiod rather than as a result of replicating seasonal reproductive environmental conditions, and that temperature plays a key role in determining the reproductive output of A. armata tetrasporophytes. These findings will enable further research to optimise the methods for seeding released tetraspores onto ropes, as well as optimising early hatchery and nursery conditions to maximise the development of gametophytes for in-sea outplanting.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bonin DR, Hawkes MW (1987) Systematics and life histories of New Zealand Bonnemaisoniaceae (Bonnemaisoniales, Rhodophyta): I. The genus Asparagopsis. N Z J Bot 25:577–590
Charrier B, Abreu MH, Araujo R, Bruhn A, Coates JC, De Clerck O, Katsaros C, Robaina RR, Wichard T (2017) Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. New Phytol 216:967–975
de Bettignies T, Wernberg T, Gurgel CFD (2018) Exploring the influence of temperature on aspects of the reproductive phenology of temperate seaweeds. Front Mar Sci 5:218
Dijoux L, Viard F, Payri C (2014) The more we search, the more we find: Discovery of a new lineage and a new species complex in the genus Asparagopsis. PLoS One 9:e103826
Duarte CM, Wu J, Xiao X, Bruhn A, Krause-Jensen D (2017) Can seaweed farming play a role in climate change mitigation and adaptation? Front Mar Sci 4:100
García-Jiménez P, Robaina RR (2012) Effects of ethylene on tetrasporogenesis in Pterocladiella capillaceae (Rhodophyta). J Phycol 48:710–715
García-Jiménez P, Robaina RR (2015) On reproduction in red algae: further research needed at the molecular level. Front Plant Sci 6:93
Garcia-Jimenez P, Robaina R (2017) Volatiles in the aquatic marine ecosystem: Ethylene and related plant hormones and sporulation in red seaweeds. In: Kumar M, Ralph P (eds) Systems Biology of Marine Ecosystems. Springer, Cham, pp 99–116
Glasson CRK, Kinley RD, de Nys R, King N, Adams SL, Packer MA, Svenson J, Eason CT, Magnusson M (2022) Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res 64:e102673
Guiry MD, Dawes CJ (1992) Daylength, temperature and nutrient control of tetrasporogenesis in Asparagopsis armata (Rhodophyta). J Exp Mar Biol Ecol 158:197–217
Ims RA (1990) The ecology and evolution of reproductive synchrony. Trends Ecol Evol 5:135–140
Jansen HM, Bernard MS, Nederlof MAJ, van der Meer IM, van der Werf A (2022) Seasonal variation in productivity, chemical composition and nutrient uptake of Ulva spp. (Chlorophyta) strains. J Appl Phycol 34:1649–1660
Jeliani ZZ, Yousefzadi M, Pour JS, Toiserkani H (2018) Growth, phytochemicals, and optimal timing of planting Gracilariopsis persica: an economic red seaweed. J Appl Phycol 30:525–533
Kai T, Nimura K, Yasui H, Mizuta H (2006) Regulation of sorus formation by auxin in Laminariales sporophyte. J Appl Phycol 18:95
Kinley RD, de Nys R, Vucko MJ, Machado L, Tomkins NW (2016) The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim Prod Sci 56:282–289
Kinley R, Martinez-Fernandez G, Matthews M, de Nys R, Magnusson M, Tomkins N (2020) Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J Clean Prod 259:e120836
Kraan S, Barrington KA (2005) Commercial farming of Asparagopsis armata (Bonnemaisoniceae, Rhodophyta) in Ireland, maintenance of an introduced species? J Appl Phycol 17:103–110
Li X, Norman HC, Kinley RD, Laurence M, Wilmot M, Bender H, de Nys R, Tomkins N (2016) Asparagopsis taxiformis decreases enteric methane production from sheep. Anim Prod Sci 58:681–688
Liu X, Bogaert K, Engelen AH, Leliaert F, Roleda MY, Clerck OD (2017) Seaweed reproductive biology: environmental and genetic controls. Bot Mar 60:89–108
Lüning K, Dieck It (1989) Environmental triggers in algal seasonality. Bot Mar 32:389–398
Machado L, Magnusson M, Paul N, Kinley R, Nys R, Tomkins N (2016) Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J Appl Phycol 28:3117–3126
Machado L, Tomkins N, Magnusson M, Midgley DJ, de Nys R, Rosewarne CP (2018) In vitro response of rumen microbiota to the antimethanogenic red macroalga Asparagopsis taxiformis. Microb Ecol 75:811–818
Mata L, Schuenhoff A, Santos R (2010) A direct comparison of the performance of the seaweed biofilters, Asparagopsis armata and Ulva rigida. J Appl Phycol 22:639–644
Mata L, Lawton RJ, Magnusson M, Andreakis N, de Nys R, Paul NA (2017) Within-species and temperature-related variation in the growth and natural products of the red alga Asparagopsis taxiformis. J Appl Phycol 29:1437–1447
Mooney-McAuley K, Edwards M, Champenois J, Gorman E (2016) Best Practice Guidelines for Seaweed Cultivation and Analysis: Public Output report [WP1A5.01] of the EnAlgae Project. EnAlgae, Swansea, UK. WP1A5.01, p 36
Nimura K, Mizuta H (2002) Inducible effects of abscisic acid on sporophyte discs from Laminaria japonica Areschoug (Laminariales, Phaeophyceae). J Appl Phycol 14:159–163
Oza RM (1977) Culture Studies on induction of tetraspores and their subsequent development in the red alga Falkenbergia rufolanosa (Harvey) Schmitz. Bot Mar 20:29–32
Pang JS, Lüning K (2004) Breaking seasonal limitation: year-round sporogenesis in the brown alga Laminaria saccharina by blocking the transport of putative sporulation inhibitors. Aquaculture 240:531–541
Pandey D, Mansouryar M, Novoa-Garrido M, Næss G, Kiron V, Hansen HH, Nielsen MO, Khanal P (2021) Nutritional and anti-methanogenic potentials of macroalgae for ruminants. In: Lei G (ed) Seaweed and microalgae as alternative sources of protein. Burleigh Dodds Science Publishing, Cambridge, pp 1–33
Pilar G-J, Olegario B-R, Rafael RR (2016) Occurrence of jasmonates during cystocarp development in the red alga Grateloupia imbricata. J Phycol 52:1085–1093
Preuss M, Nelson WA, D’Archino R (2022) Cryptic diversity and phylogeographic patterns in the Asparagopsis armata species complex (Bonnemaisoniales, Rhodophyta) from New Zealand. Phycologia 61:89–96
Redmond S, Green-Gavrielidis L, Yarish C, Kim J, Neefus C (2013) New England Seaweed Culture Handbook: Nursery Systems. Connecticut Sea Grant, Connecticut, USA
Richardson JTE (2011) Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev 6:135–147
Roque BM, Salwen JK, Kinley R, Kebreab E (2019) Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J Clean Prod 234:132–138
Roque BM, Venegas M, Kinley RD, de Nys R, Duarte TL, Yang X, Kebreab E (2021) Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS One 16:e0247820
Rule M, Wernberg T, Kendrick G, Rule M (2013) Reproductive synchrony in a habitat-forming kelp and its relationship with environmental conditions. Mar Biol 160:119–126
Sacramento AT, García-Jiménez P, Robaina RR (2007) The polyamine spermine induces cystocarp development in the seaweed Grateloupia (Rhodophyta). Plant Growth Regul 53:147–154
Sea Temperature Info (2022) Water temperature in Goat Island. https://seatemperature.info/new-zealand/goat-island-water-temperature.html.
Stirk WA, Van Staden J (2014) Plant growth regulators in seaweeds: occurrence, regulation and functions. Adv Bot Res 71:125–159
Sunrise and sunset (2020) Sunrise and sunset Auckland, New Zealand. https://www.sunrise-and-sunset.com/en/sun/new-zealand/auckland
Taelman SE, Champenois J, Edwards MD, De Meester S, Dewulf J (2015) Comparative environmental life cycle assessment of two seaweed cultivation systems in North West Europe with a focus on quantifying sea surface occupation. Algal Res 11:173–183
Uji T, Endo H, Mizuta H (2020) Sexual reproduction via a 1-aminocyclopropane-1-carboxylic acid-dependent pathway through redox modulation in the marine red alga Pyropia yezoensis (Rhodophyta). Front Plant Sci 11:60
Vijn S, Compart DP, Dutta N, Foukis A, Hess M, Hristov AN, Kalscheur KF, Kebreab E, Nuzhdin SV, Price NN, Sun Y, Tricarico JM, Turzillo A, Weisbjerg MR, Yarish C, Kurt TD (2020) Key Considerations for the use of seaweed to reduce enteric methane emissions from cattle. Front Vet Sci 7:e597430
Watson L, Dring M (2011) Business plan for the establishment of a seaweed hatchery and grow-out farm (Part 2). Irish Sea Fisheries Board, Dublin, Ireland. PBA/SW/07/001 (01), p 39
Werner A, Dring M (2011) Aquaculture explained No. 27. Cultivating Palmaria palmata. Irish Sea Fisheries Board, Dublin, Ireland. PBA/SW/07/001 (27), p 47
World Data Info (2022) Sunrise and sunset data. https://www.worlddata.info/
Wright JT, Kennedy EJ, de Nys R, Tatsumi M (2022) Asexual propagation of Asparagopsis armata gametophytes: fragmentation, regrowth and attachment mechanisms for sea-based cultivation. J Appl Phycol 34:2135–2144
Acknowledgements
This research is part of the Entrepreneurial Universities Macroalgal Biotechnologies Programme, jointly funded by the University of Waikato and the New Zealand Tertiary Education Commission. Funding for this project was also provided by Sea Forest Ltd. We sincerely thank Maro Guy for creating the schematic life cycle diagram. We also thank Holly Robertson and Nethmie Jayasooriya for their assistance in maintaining the experimental culture cabinet setup, as well as Andrew Cole for his insightful suggestions and knowledge.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research is part of the Entrepreneurial Universities Macroalgal Biotechnologies Programme, jointly funded by the University of Waikato and the New Zealand Tertiary Education Commission. Funding for this project was also provided by Sea Forest Ltd.
Author information
Authors and Affiliations
Contributions
Alisa Mihaila: Methodology, data collection, formal analysis, writing – original draft, review & editing; Rebecca Lawton: Conceptualisation, methodology, supervision, writing – review and editing; Christopher Glasson: Conceptualisation, methodology, supervision, writing – review and editing; Marie Magnusson: Conceptualisation, funding acquisition, methodology, supervision, writing – original draft, review and editing.
Corresponding author
Ethics declarations
Competing interests
Data presented here form part of Provisional patent 197290PRV, filed by SeaForest of May 12, 2022. Magnusson sits on the advisory board of SeaForest Ltd who partially funded this research, however, SeaForest had no involvement in the conduct of the research; study design; the collection, analysis and interpretation of data; or in the writing of the report.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mihaila, A.A., Lawton, R.J., Glasson, C.R.K. et al. Early hatchery protocols for tetrasporogenesis of the antimethanogenic seaweed Asparagopsis armata. J Appl Phycol 35, 2323–2335 (2023). https://doi.org/10.1007/s10811-023-03029-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03029-5